Synapse-specific structural plasticity that protects and refines local circuits during LTP and LTD
- PMID: 38853547
- PMCID: PMC11529630
- DOI: 10.1098/rstb.2023.0224
Synapse-specific structural plasticity that protects and refines local circuits during LTP and LTD
Abstract
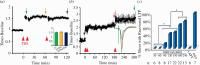
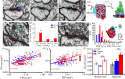
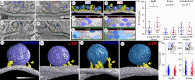
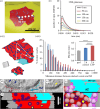
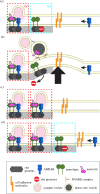
Synapses form trillions of connections in the brain. Long-term potentiation (LTP) and long-term depression (LTD) are cellular mechanisms vital for learning that modify the strength and structure of synapses. Three-dimensional reconstruction from serial section electron microscopy reveals three distinct pre- to post-synaptic arrangements: strong active zones (AZs) with tightly docked vesicles, weak AZs with loose or non-docked vesicles, and nascent zones (NZs) with a postsynaptic density but no presynaptic vesicles. Importantly, LTP can be temporarily saturated preventing further increases in synaptic strength. At the onset of LTP, vesicles are recruited to NZs, converting them to AZs. During recovery of LTP from saturation (1-4 h), new NZs form, especially on spines where AZs are most enlarged by LTP. Sentinel spines contain smooth endoplasmic reticulum (SER), have the largest synapses and form clusters with smaller spines lacking SER after LTP recovers. We propose a model whereby NZ plasticity provides synapse-specific AZ expansion during LTP and loss of weak AZs that drive synapse shrinkage during LTD. Spine clusters become functionally engaged during LTP or disassembled during LTD. Saturation of LTP or LTD probably acts to protect recently formed memories from ongoing plasticity and may account for the advantage of spaced over massed learning. This article is part of a discussion meeting issue 'Long-term potentiation: 50 years on'.
Keywords: long-term depression; long-term potentiation; spaced learning; synapse; ultrastructure.
Conflict of interest statement
We declare we have no competing interests.
Figures



References
-
- Ramón Y Y, Cajal S. 1891. Sur la structure de l’ecorce cerebrale de quelques mammiferes. Cellule 7 , 124–176.
-
- Ramón Y, Cajal S. 1896. Las espinas colaterales de las celulas del cerebro tenidas por el azul de metileno. Revista Trimestral Microgratica 1 , 123–136.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

