Cage effects on synaptic plasticity and its modulation in a mouse model of fragile X syndrome
- PMID: 38853552
- PMCID: PMC11343313
- DOI: 10.1098/rstb.2023.0484
Cage effects on synaptic plasticity and its modulation in a mouse model of fragile X syndrome
Abstract
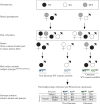
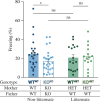
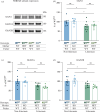
Fragile X syndrome (FXS) is characterized by impairments in executive function including different types of learning and memory. Long-term potentiation (LTP), thought to underlie the formation of memories, has been studied in the Fmr1 mouse model of FXS. However, there have been many discrepancies in the literature with inconsistent use of littermate and non-littermate Fmr1 knockout (KO) and wild-type (WT) control mice. Here, the influence of the breeding strategy (cage effect) on short-term potentiation (STP), LTP, contextual fear conditioning (CFC), expression of N-methyl-d-aspartate receptor (NMDAR) subunits and the modulation of NMDARs, were examined. The largest deficits in STP, LTP and CFC were found in KO mice compared with non-littermate WT. However, the expression of NMDAR subunits was unchanged in this comparison. Rather, NMDAR subunit (GluN1, 2A, 2B) expression was sensitive to the cage effect, with decreased expression in both WT and KO littermates compared with non-littermates. Interestingly, an NMDAR-positive allosteric modulator, UBP714, was only effective in potentiating the induction of LTP in non-littermate KO mice and not the littermate KO mice. These results suggest that commonly studied phenotypes in Fmr1 KOs are sensitive to the cage effect and therefore the breeding strategy may contribute to discrepancies in the literature.This article is part of a discussion meeting issue 'Long-term potentiation: 50 years on'.
Keywords: CFC; Fmr1; LTP; NMDAR PAM; STP; littermate syndrome.
Conflict of interest statement
G.L.C. and D.E.J. are on the Scientific Advisory Board of Hello Bio, a supplier of UBP714.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
