Adiponectin rescues synaptic plasticity in the dentate gyrus of a mouse model of Fragile X Syndrome
- PMID: 38853554
- PMCID: PMC11343265
- DOI: 10.1098/rstb.2023.0221
Adiponectin rescues synaptic plasticity in the dentate gyrus of a mouse model of Fragile X Syndrome
Abstract
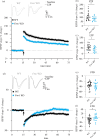
Fragile X syndrome (FXS) is the most common inherited cause of intellectual disability and is the leading known single-gene cause of autism spectrum disorder. Patients with FXS display varied behavioural deficits that include mild to severe cognitive impairments in addition to mood disorders. Currently, there is no cure for this condition; however, there is an emerging focus on therapies that inhibit mechanistic target of rapamycin (mTOR)-dependent protein synthesis owing to the clinical effectiveness of metformin for alleviating some behavioural symptoms in FXS. Adiponectin (APN) is a neurohormone that is released by adipocytes and provides an alternative means to inhibit mTOR activation in the brain. In these studies, we show that Fmr1 knockout mice, like patients with FXS, show reduced levels of circulating APN and that both long-term potentiation (LTP) and long-term depression (LTD) in the dentate gyrus (DG) are impaired. Brief (20 min) incubation of hippocampal slices in APN (50 nM) was able to rescue both LTP and LTD in the DG and increased both the surface expression and phosphorylation of GluA1 receptors. These results provide evidence for reduced APN levels in FXS playing a role in decreasing bidirectional synaptic plasticity and show that therapies which enhance APN levels may have therapeutic potential for this and related conditions.This article is part of a discussion meeting issue 'Long-term potentiation: 50 years on'.
Keywords: adiponectin; dentate gyrus; fragile X syndrome; hippocampus; long-term potentiation; mTOR.
Conflict of interest statement
We declare we have no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
