Differential effectiveness of dietary zinc supplementation with autism-related behaviours in Shank2 knockout mice
- PMID: 38853567
- PMCID: PMC11343228
- DOI: 10.1098/rstb.2023.0230
Differential effectiveness of dietary zinc supplementation with autism-related behaviours in Shank2 knockout mice
Abstract
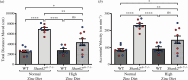
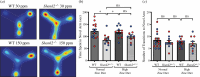
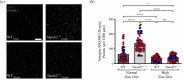
The family of SHANK proteins have been shown to be critical in regulating glutamatergic synaptic structure, function and plasticity. SHANK variants are also prevalent in autism spectrum disorders (ASDs), where glutamatergic synaptopathology has been shown to occur in multiple ASD mouse models. Our previous work has shown that dietary zinc in Shank3-/- and Tbr1+/- ASD mouse models can reverse or prevent ASD behavioural and synaptic deficits. Here, we have examined whether dietary zinc can influence behavioural and synaptic function in Shank2-/- mice. Our data show that dietary zinc supplementation can reverse hyperactivity and social preference behaviour in Shank2-/- mice, but it does not alter deficits in working memory. Consistent with this, at the synaptic level, deficits in NMDA/AMPA receptor-mediated transmission are also not rescued by dietary zinc. In contrast to other ASD models examined, we observed that SHANK3 protein was highly expressed at the synapses of Shank2-/- mice and that dietary zinc returned these to wild-type levels. Overall, our data show that dietary zinc has differential effectiveness in altering ASD behaviours and synaptic function across ASD mouse models even within the Shank family. This article is part of a discussion meeting issue 'Long-term potentiation: 50 years on'.
Keywords: autism; shank; synapse; zinc.
Conflict of interest statement
We declare we have no competing interests.
Figures

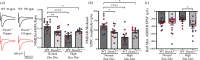
References
-
- Chilian B, Abdollahpour H, Bierhals T, Haltrich I, Fekete G, Nagel I, Rosenberger G, Kutsche K. 2013. Dysfunction of SHANK2 and CHRNA7 in a patient with intellectual disability and language impairment supports genetic epistasis of the two loci. Clin. Genet. 84, 560–565. (10.1111/cge.12105) - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

