Cytomegalovirus Antiviral Resistance Among Kidney Transplant Recipients in a Phase 3 Trial of Letermovir vs Valganciclovir Prophylaxis
- PMID: 38853607
- PMCID: PMC11646593
- DOI: 10.1093/infdis/jiae287
Cytomegalovirus Antiviral Resistance Among Kidney Transplant Recipients in a Phase 3 Trial of Letermovir vs Valganciclovir Prophylaxis
Abstract
Background: In a phase 3 trial, letermovir was noninferior to valganciclovir for cytomegalovirus (CMV) disease prophylaxis in kidney transplant recipients who were CMV-seronegative and received kidneys from donors who were CMV-seropositive. Genotypic antiviral resistance and CMV glycoprotein B (gB) genotype are reported.
Methods: Plasma samples with detectable CMV DNA were sequenced for the presence of known letermovir and valganciclovir resistance-associated amino acid substitutions (RASs) encoded by CMV gene regions (UL51, UL54, UL56, UL89, UL97) and prevalence of gB (UL55) genotypes (gB1-gB5).
Results: Among participants, 84 of 292 (letermovir) and 93 of 297 (valganciclovir) had evaluable data for ≥1 gene target. Letermovir RASs were not detected in participants who received letermovir prophylaxis; however, 3 had valganciclovir RASs (pUL97). Twelve participants who received valganciclovir prophylaxis had valganciclovir RASs (pUL54, pUL97), and 1 who did not receive letermovir during the trial had letermovir RASs (pUL56). All but 1 participant responded to valganciclovir treatment irrespective of breakthrough CMV DNAemia or frequency of RASs. gB1 was the most frequent genotype across all participants and subgroups.
Conclusions: Letermovir RASs were not detected with letermovir prophylaxis, supporting a low risk for development of resistance in kidney transplant recipients who were CMV-seronegative and received kidneys from donors who were CMV-seropositive.
Clinical trials registration: ClinicalTrials.gov, NCT03443869; EudraCT, 2017-001055-30.
Keywords: cytomegalovirus; kidney transplant recipient; letermovir; prophylaxis; resistance.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors are current or former employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, USA who may own stock and/or hold stock options in Merck & Co, Inc, Rahway, NJ, USA. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

 letermovir prophylaxis;
letermovir prophylaxis;  valganciclovir treatment. Symbols reflect the outcome from genotypic analysis:
valganciclovir treatment. Symbols reflect the outcome from genotypic analysis:  sample not collected;
sample not collected;  RAS detected;
RAS detected;  sequencing failed. *Confirmed CMV disease.
sequencing failed. *Confirmed CMV disease.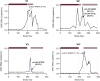
 valganciclovir prophylaxis;
valganciclovir prophylaxis;  valganciclovir/ganciclovir treatment;
valganciclovir/ganciclovir treatment;  foscarnet treatment. Symbols reflect the outcome from genotypic analysis:
foscarnet treatment. Symbols reflect the outcome from genotypic analysis:  sample not collected;
sample not collected;  RAS detected;
RAS detected;  RAS not detected;
RAS not detected;  sequencing failed. *Confirmed CMV disease.
sequencing failed. *Confirmed CMV disease.
 valganciclovir prophylaxis;
valganciclovir prophylaxis;  valganciclovir/ganciclovir treatment. Symbols reflect the outcome from genotypic analysis:
valganciclovir/ganciclovir treatment. Symbols reflect the outcome from genotypic analysis:  sample not collected;
sample not collected;  RAS detected;
RAS detected;  RAS not detected;
RAS not detected;  sequencing failed. *Confirmed CMV disease.
sequencing failed. *Confirmed CMV disease.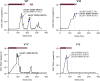
 valganciclovir prophylaxis;
valganciclovir prophylaxis;  valganciclovir/ganciclovir treatment;
valganciclovir/ganciclovir treatment;  letermovir treatment;
letermovir treatment;  foscarnet treatment. Symbols reflect the outcome from genotypic analysis:
foscarnet treatment. Symbols reflect the outcome from genotypic analysis:  sample not collected;
sample not collected;  RAS detected;
RAS detected;  RAS not detected. *Confirmed CMV disease. †RAS not detected upon repeat next-generation sequencing.
RAS not detected. *Confirmed CMV disease. †RAS not detected upon repeat next-generation sequencing.References
-
- Valcyte (valganciclovir). US prescribing information. South San Francisco, CA: Genentech USA, Inc, 2021. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/021304.s17_222.... Accessed 19 December 2023.
-
- Kotton CN, Kumar D, Caliendo AM, et al. The Third International Consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2018; 102:900–31. - PubMed
-
- Prevymis (letermovir). US prescribing information. Rahway, NJ: Merck Sharp & Dohme LLC, 2023. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/209939s011,209.... Accessed 19 December 2023.
-
- Kleiboeker SB. Prevalence of cytomegalovirus antiviral drug resistance in transplant recipients. Antiviral Res 2023; 215:105623. - PubMed

