This is a preprint.
Phosphorylation of VapB antitoxins affects intermolecular interactions to regulate VapC toxin activity in Mycobacterium tuberculosis
- PMID: 38853858
- PMCID: PMC11160731
- DOI: 10.1101/2024.05.30.596101
Phosphorylation of VapB antitoxins affects intermolecular interactions to regulate VapC toxin activity in Mycobacterium tuberculosis
Update in
-
Phosphorylation of VapB antitoxins affects intermolecular interactions to regulate VapC toxin activity in Mycobacterium tuberculosis.J Bacteriol. 2024 Oct 24;206(10):e0023324. doi: 10.1128/jb.00233-24. Epub 2024 Sep 24. J Bacteriol. 2024. PMID: 39315797 Free PMC article.
Abstract
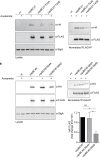
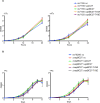
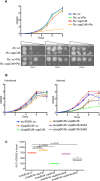
Toxin-antitoxin modules are present in many bacterial pathogens. The VapBC family is particularly abundant in members of the Mycobacterium tuberculosis complex, with 50 modules present in the M. tuberculosis genome. In type IIA modules the VapB antitoxin protein binds to and inhibits the activity of the co-expressed cognate VapC toxin protein. VapB proteins also bind to promoter region sequences and repress expression of the vapB-vapC operon. Though VapB-VapC interactions can control the amount of free VapC toxin in the bacterial cell, the mechanisms that affect this interaction are poorly understood. Based on our recent finding of Ser/Thr phosphorylation of VapB proteins in M. tuberculosis, we substituted phosphomimetic or phosphoablative amino acids at the phosphorylation sites of two VapB proteins. We found that phosphomimetic substitution of VapB27 and VapB46 resulted in decreased interaction with their respective cognate VapC proteins, whereas phosphoablative substitution did not alter binding. Similarly, we determined that phosphomimetic substitution interfered with VapB binding to promoter region DNA sequences. Both decreased VapB-VapC interaction and decreased VapB repression of vapB-vapC operon transcription would result in increased free VapC in the M. tuberculosis cell. M. tuberculosis strains expressing vapB46-vapC46 constructs containing a phosphoablative vapB mutation resulted in lower toxicity compared to a strain expressing native vapB46, whereas similar or greater toxicity was observed in the strain expressing the phosphomimetic vapB mutation. These results identify a novel mechanism by which VapC toxicity activity can be regulated by VapB phosphorylation, potentially in response to extracytoplasmic as well as intracellular signals.
Keywords: Mycobacterium tuberculosis; Toxin-antitoxin; VapB; VapC; bacterial stress response; latency; persistence; protein phosphorylation.
Figures


Similar articles
-
Phosphorylation of VapB antitoxins affects intermolecular interactions to regulate VapC toxin activity in Mycobacterium tuberculosis.J Bacteriol. 2024 Oct 24;206(10):e0023324. doi: 10.1128/jb.00233-24. Epub 2024 Sep 24. J Bacteriol. 2024. PMID: 39315797 Free PMC article.
-
VapC toxins from Mycobacterium tuberculosis are ribonucleases that differentially inhibit growth and are neutralized by cognate VapB antitoxins.PLoS One. 2011;6(6):e21738. doi: 10.1371/journal.pone.0021738. Epub 2011 Jun 29. PLoS One. 2011. PMID: 21738782 Free PMC article.
-
Characterization of the Deep-Sea Streptomyces sp. SCSIO 02999 Derived VapC/VapB Toxin-Antitoxin System in Escherichia coli.Toxins (Basel). 2016 Jul 1;8(7):195. doi: 10.3390/toxins8070195. Toxins (Basel). 2016. PMID: 27376329 Free PMC article.
-
Deciphering the role of VapBC toxin-antitoxin systems in Mycobacterium tuberculosis stress adaptation.Future Microbiol. 2024;19(18):1587-1599. doi: 10.1080/17460913.2024.2412447. Epub 2024 Oct 21. Future Microbiol. 2024. PMID: 39431307 Review.
-
Focused Overview of Mycobacterium tuberculosis VapBC Toxin-Antitoxin Systems Regarding Their Structural and Functional Aspects: Including Insights on Biomimetic Peptides.Biomimetics (Basel). 2023 Sep 6;8(5):412. doi: 10.3390/biomimetics8050412. Biomimetics (Basel). 2023. PMID: 37754163 Free PMC article. Review.
References
-
- World Health Organization. 2023. Global tuberculosis report 2023., Geneva.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
