This is a preprint.
Single neuron contributions to the auditory brainstem EEG
- PMID: 38853863
- PMCID: PMC11160769
- DOI: 10.1101/2024.05.29.596509
Single neuron contributions to the auditory brainstem EEG
Update in
-
Single Neuron Contributions to the Auditory Brainstem EEG.J Neurosci. 2025 May 28;45(22):e1139242025. doi: 10.1523/JNEUROSCI.1139-24.2025. J Neurosci. 2025. PMID: 40262897 Free PMC article.
Abstract
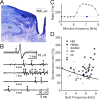
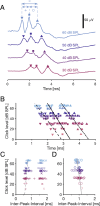
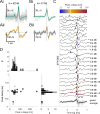
The auditory brainstem response (ABR) is an acoustically evoked EEG potential that is an important diagnostic tool for hearing loss, especially in newborns. The ABR originates from the response sequence of auditory nerve and brainstem nuclei, and a click-evoked ABR typically shows three positive peaks ('waves') within the first six milliseconds. However, an assignment of the waves of the ABR to specific sources is difficult, and a quantification of contributions to the ABR waves is not available. Here, we exploit the large size and physical separation of the barn owl first-order cochlear nucleus magnocellularis (NM) to estimate single-cell contributions to the ABR. We simultaneously recorded NM neurons' spikes and the EEG in owls of both sexes, and found that ≳ 5,000 spontaneous single-cell spikes are necessary to isolate a significant spike-triggered average response at the EEG electrode. An average single-neuron contribution to the ABR was predicted by convolving the spike-triggered average with the cell's peri-stimulus time histogram. Amplitudes of predicted contributions of single NM cells typically reached 32.9 ± 1.1 nV (mean ± SE, range: 2.5 - 162.7 nV), or 0.07 ± 0.02% (median ± SE; range from 0.01% to 1%) of the ABR amplitude. The time of the predicted peak coincided best with the peak of the ABR wave II, independent of the click sound level. Our results suggest that individual neurons' contributions to an EEG can vary widely, and that wave II of the ABR is shaped by NM units.
Significance statement: The auditory brainstem response (ABR) is a scalp potential used for the diagnosis of hearing loss, both clinically and in research. We investigated the contribution of single action potentials from auditory brainstem neurons to the ABR and provide direct evidence that action potentials recorded in a first order auditory nucleus, and their EEG contribution, coincide with wave II of the ABR. The study also shows that the contribution of single cells varies strongly across the population.
Conflict of interest statement
Conflict of interest: No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Abdi H, Bonferroni and šidák corrections for multiple comparisons In: Encyclopedia of measurement and statistics, edited by Salkin N. Sage, Thousand Oaks, CA, US, 2007. p. 103 – 107.
-
- Achor LJ, Starr A. Auditory brain stem responses in the cat. I. intracranial and extracranial recordings. Electroencephalogr. Clin. Neurophysiol. 48: 154–173, 1980. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
