This is a preprint.
Malaria parasites require a divergent heme oxygenase for apicoplast gene expression and biogenesis
- PMID: 38853871
- PMCID: PMC11160694
- DOI: 10.1101/2024.05.30.596652
Malaria parasites require a divergent heme oxygenase for apicoplast gene expression and biogenesis
Update in
-
Malaria parasites require a divergent heme oxygenase for apicoplast gene expression and biogenesis.Elife. 2024 Dec 11;13:RP100256. doi: 10.7554/eLife.100256. Elife. 2024. PMID: 39660822 Free PMC article.
Abstract
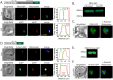
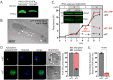
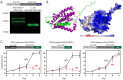
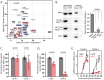
Malaria parasites have evolved unusual metabolic adaptations that specialize them for growth within heme-rich human erythrocytes. During blood-stage infection, Plasmodium falciparum parasites internalize and digest abundant host hemoglobin within the digestive vacuole. This massive catabolic process generates copious free heme, most of which is biomineralized into inert hemozoin. Parasites also express a divergent heme oxygenase (HO)-like protein (PfHO) that lacks key active-site residues and has lost canonical HO activity. The cellular role of this unusual protein that underpins its retention by parasites has been unknown. To unravel PfHO function, we first determined a 2.8 Å-resolution X-ray structure that revealed a highly α-helical fold indicative of distant HO homology. Localization studies unveiled PfHO targeting to the apicoplast organelle, where it is imported and undergoes N-terminal processing but retains most of the electropositive transit peptide. We observed that conditional knockdown of PfHO was lethal to parasites, which died from defective apicoplast biogenesis and impaired isoprenoid-precursor synthesis. Complementation and molecular-interaction studies revealed an essential role for the electropositive N-terminus of PfHO, which selectively associates with the apicoplast genome and enzymes involved in nucleic acid metabolism and gene expression. PfHO knockdown resulted in a specific deficiency in levels of apicoplast-encoded RNA but not DNA. These studies reveal an essential function for PfHO in apicoplast maintenance and suggest that Plasmodium repurposed the conserved HO scaffold from its canonical heme-degrading function in the ancestral chloroplast to fulfill a critical adaptive role in organelle gene expression.
Figures

Similar articles
-
Malaria parasites require a divergent heme oxygenase for apicoplast gene expression and biogenesis.Elife. 2024 Dec 11;13:RP100256. doi: 10.7554/eLife.100256. Elife. 2024. PMID: 39660822 Free PMC article.
-
CaaX-Like Protease of Cyanobacterial Origin Is Required for Complex Plastid Biogenesis in Malaria Parasites.mBio. 2020 Oct 6;11(5):e01492-20. doi: 10.1128/mBio.01492-20. mBio. 2020. PMID: 33024034 Free PMC article.
-
Identification of a divalent metal transporter required for cellular iron metabolism in malaria parasites.Proc Natl Acad Sci U S A. 2024 Nov 5;121(45):e2411631121. doi: 10.1073/pnas.2411631121. Epub 2024 Oct 28. Proc Natl Acad Sci U S A. 2024. PMID: 39467134 Free PMC article.
-
Biogenesis and maintenance of the apicoplast in model apicomplexan parasites.Parasitol Int. 2021 Apr;81:102270. doi: 10.1016/j.parint.2020.102270. Epub 2020 Dec 13. Parasitol Int. 2021. PMID: 33321224 Review.
-
The apicoplast: a plastid in Plasmodium falciparum and other Apicomplexan parasites.Int Rev Cytol. 2003;224:57-110. doi: 10.1016/s0074-7696(05)24003-2. Int Rev Cytol. 2003. PMID: 12722949 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
