This is a preprint.
Enhancing the Diagnostic Utility of ASL Imaging in Temporal Lobe Epilepsy through FlowGAN: An ASL to PET Image Translation Framework
- PMID: 38853910
- PMCID: PMC11160820
- DOI: 10.1101/2024.05.28.24308027
Enhancing the Diagnostic Utility of ASL Imaging in Temporal Lobe Epilepsy through FlowGAN: An ASL to PET Image Translation Framework
Abstract
Background and significance: Positron Emission Tomography (PET) using fluorodeoxyglucose (FDG-PET) is a standard imaging modality for detecting areas of hypometabolism associated with the seizure onset zone (SOZ) in temporal lobe epilepsy (TLE). However, FDG-PET is costly and involves the use of a radioactive tracer. Arterial Spin Labeling (ASL) offers an MRI-based quantification of cerebral blood flow (CBF) that could also help localize the SOZ, but its performance in doing so, relative to FDG-PET, is limited. In this study, we seek to improve ASL's diagnostic performance by developing a deep learning framework for synthesizing FDG-PET-like images from ASL and structural MRI inputs.
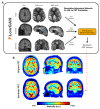
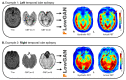
Methods: We included 68 epilepsy patients, out of which 36 had well lateralized TLE. We compared the coupling between FDG-PET and ASL CBF values in different brain regions, as well as the asymmetry of these values across the brain. We additionally assessed each modality's ability to lateralize the SOZ across brain regions. Using our paired PET-ASL data, we developed FlowGAN, a generative adversarial neural network (GAN) that synthesizes PET-like images from ASL and T1-weighted MRI inputs. We tested our synthetic PET images against the actual PET images of subjects to assess their ability to reproduce clinically meaningful hypometabolism and asymmetries in TLE.
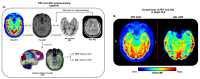
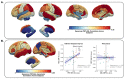
Results: We found variable coupling between PET and ASL CBF values across brain regions. PET and ASL had high coupling in neocortical temporal and frontal brain regions (Spearman's r > 0.30, p < 0.05) but low coupling in mesial temporal structures (Spearman's r < 0.30, p > 0.05). Both whole brain PET and ASL CBF asymmetry values provided good separability between left and right TLE subjects, but PET (AUC = 0.96, 95% CI: [0.88, 1.00]) outperformed ASL (AUC = 0.81; 95% CI: [0.65, 0.96]). FlowGAN-generated images demonstrated high structural similarity to actual PET images (SSIM = 0.85). Globally, asymmetry values were better correlated between synthetic PET and original PET than between ASL CBF and original PET, with a mean correlation increase of 0.15 (95% CI: [0.07, 0.24], p<0.001, Cohen's d = 0.91). Furthermore, regions that had poor ASL-PET correlation (e.g. mesial temporal structures) showed the greatest improvement with synthetic PET images.
Conclusions: FlowGAN improves ASL's diagnostic performance, generating synthetic PET images that closely mimic actual FDG-PET in depicting hypometabolism associated with TLE. This approach could improve non-invasive SOZ localization, offering a promising tool for epilepsy presurgical assessment. It potentially broadens the applicability of ASL in clinical practice and could reduce reliance on FDG-PET for epilepsy and other neurological disorders.
Conflict of interest statement
Competing Interests Thomas Campbell Arnold is an employee of Subtle Medical, but this work is unrelated to his work at the company. He contributed to this work during his time at the University of Pennsylvania. The rest of the authors report no competing or financial interests.
Figures

Similar articles
-
Exploring ASL perfusion MRI as a substitutive modality for 18F-FDG PET in determining the laterality of mesial temporal lobe epilepsy.Neurol Sci. 2024 May;45(5):2223-2243. doi: 10.1007/s10072-023-07188-8. Epub 2023 Nov 23. Neurol Sci. 2024. PMID: 37994963
-
Detection of Crossed Cerebellar Diaschisis in Intractable Epilepsy Using Integrated 18F-FDG PET/MR Imaging.Cerebellum. 2024 Dec 21;24(1):18. doi: 10.1007/s12311-024-01759-x. Cerebellum. 2024. PMID: 39707060
-
Comparison between simultaneously acquired arterial spin labeling and 18F-FDG PET in mesial temporal lobe epilepsy assisted by a PET/MR system and SEEG.Neuroimage Clin. 2018 Jun 7;19:824-830. doi: 10.1016/j.nicl.2018.06.008. eCollection 2018. Neuroimage Clin. 2018. PMID: 30013926 Free PMC article.
-
Arterial spin labeling (ASL-MRI) versus fluorodeoxyglucose-PET (FDG-PET) in diagnosing dementia: a systematic review and meta-analysis.BMC Neurol. 2023 Oct 24;23(1):385. doi: 10.1186/s12883-023-03432-y. BMC Neurol. 2023. PMID: 37875879 Free PMC article.
-
Neuronuclear assessment of patients with epilepsy.Semin Nucl Med. 2008 Jul;38(4):227-39. doi: 10.1053/j.semnuclmed.2008.02.004. Semin Nucl Med. 2008. PMID: 18514079 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
