This is a preprint.
Oligomeric amyloid beta prevents myelination in a clusterin-dependent manner
- PMID: 38853911
- PMCID: PMC11160922
- DOI: 10.21203/rs.3.rs-4415143/v1
Oligomeric amyloid beta prevents myelination in a clusterin-dependent manner
Abstract
Background: White matter loss is a well-documented phenomenon in Alzheimer's disease (AD) patients that has been recognized for decades. However, the underlying reasons for the failure of oligodendrocyte progenitor cells (OPCs) to repair myelin deficits in these patients remain elusive. A single nucleotide polymorphism (SNP) in Clusterin has been identified as a risk factor for late-onset Alzheimer's disease and linked to a decrease in white matter integrity in healthy adults, but its specific role in oligodendrocyte function and myelin maintenance in Alzheimer's disease pathology remains unclear.
Methods: To investigate the impact of Clusterin on OPCs in the context of Alzheimer's disease, we employed a combination of immunofluorescence and transmission electron microscopy techniques, primary culture of OPCs, and an animal model of Alzheimer's disease.
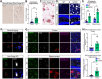
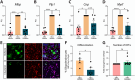
Results: Our findings demonstrate that Clusterin, a risk factor for late-onset AD, is produced by OPCs and inhibits their differentiation into oligodendrocytes. Specifically, we observed upregulation of Clusterin in OPCs in the 5xFAD mouse model of AD. We also found that the phagocytosis of debris, including amyloid beta (Aβ), myelin, and apoptotic cells leads to the upregulation of Clusterin in OPCs. In vivo experiments confirmed that Aβ oligomers stimulate Clusterin upregulation and that OPCs are capable of phagocytosing Aβ. Furthermore, we discovered that Clusterin significantly inhibits OPC differentiation and hinders the production of myelin proteins. Finally, we demonstrate that Clusterin inhibits OPC differentiation by reducing the production of IL-9 by OPCs.
Conclusion: Our data suggest that Clusterin may play a key role in the impaired myelin repair observed in AD and could serve as a promising therapeutic target for addressing AD-associated cognitive decline.
Keywords: Alzheimer’s Disease; IL-9; Oligodendrocyte progenitor cells; clusterin; myelin.
Conflict of interest statement
Competing interest The authors have no competing interests.
Figures




References
-
- Association As. 2021 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2021;17:327–406. - PubMed
-
- Roher AE, Weiss N, Kokjohn TA, Kuo Y-M, Kalback W, Anthony J, Watson D, Luehrs DC, Sue L, Walker D, et al. Increased Aβ Peptides and Reduced Cholesterol and Myelin Proteins Characterize White Matter Degeneration in Alzheimer’s Disease. Biochemistry. 2002;41:11080–90. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

