This is a preprint.
Ancient eukaryotic protein interactions illuminate modern genetic traits and disorders
- PMID: 38853926
- PMCID: PMC11160598
- DOI: 10.1101/2024.05.26.595818
Ancient eukaryotic protein interactions illuminate modern genetic traits and disorders
Abstract
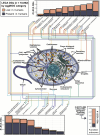
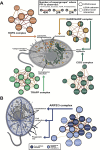
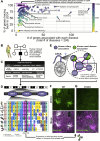
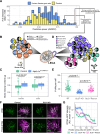
All eukaryotes share a common ancestor from roughly 1.5 - 1.8 billion years ago, a single-celled, swimming microbe known as LECA, the Last Eukaryotic Common Ancestor. Nearly half of the genes in modern eukaryotes were present in LECA, and many current genetic diseases and traits stem from these ancient molecular systems. To better understand these systems, we compared genes across modern organisms and identified a core set of 10,092 shared protein-coding gene families likely present in LECA, a quarter of which are uncharacterized. We then integrated >26,000 mass spectrometry proteomics analyses from 31 species to infer how these proteins interact in higher-order complexes. The resulting interactome describes the biochemical organization of LECA, revealing both known and new assemblies. We analyzed these ancient protein interactions to find new human gene-disease relationships for bone density and congenital birth defects, demonstrating the value of ancestral protein interactions for guiding functional genetics today.
Figures



Similar articles
-
Comparative genomics of eukaryotic small nucleolar RNAs reveals deep evolutionary ancestry amidst ongoing intragenomic mobility.BMC Evol Biol. 2012 Sep 15;12:183. doi: 10.1186/1471-2148-12-183. BMC Evol Biol. 2012. PMID: 22978381 Free PMC article.
-
The spread of the first introns in proto-eukaryotic paralogs.Commun Biol. 2022 May 19;5(1):476. doi: 10.1038/s42003-022-03426-5. Commun Biol. 2022. PMID: 35589959 Free PMC article.
-
Mosaic origin of the eukaryotic kinetochore.Proc Natl Acad Sci U S A. 2019 Jun 25;116(26):12873-12882. doi: 10.1073/pnas.1821945116. Epub 2019 May 24. Proc Natl Acad Sci U S A. 2019. PMID: 31127038 Free PMC article.
-
Origin and evolution of spliceosomal introns.Biol Direct. 2012 Apr 16;7:11. doi: 10.1186/1745-6150-7-11. Biol Direct. 2012. PMID: 22507701 Free PMC article. Review.
-
Concepts of the last eukaryotic common ancestor.Nat Ecol Evol. 2019 Mar;3(3):338-344. doi: 10.1038/s41559-019-0796-3. Epub 2019 Feb 18. Nat Ecol Evol. 2019. PMID: 30778187 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
