This is a preprint.
Cortical determinants of loudness perception and auditory hypersensitivity
- PMID: 38853938
- PMCID: PMC11160727
- DOI: 10.1101/2024.05.30.596691
Cortical determinants of loudness perception and auditory hypersensitivity
Abstract
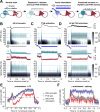
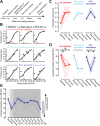
Parvalbumin-expressing inhibitory neurons (PVNs) stabilize cortical network activity, generate gamma rhythms, and regulate experience-dependent plasticity. Here, we observed that activation or inactivation of PVNs functioned like a volume knob in the mouse auditory cortex (ACtx), turning neural and behavioral classification of sound level up or down over a 20dB range. PVN loudness adjustments were "sticky", such that a single bout of 40Hz PVN stimulation sustainably suppressed ACtx sound responsiveness, potentiated feedforward inhibition, and behaviorally desensitized mice to loudness. Sensory sensitivity is a cardinal feature of autism, aging, and peripheral neuropathy, prompting us to ask whether PVN stimulation can persistently desensitize mice with ACtx hyperactivity, PVN hypofunction, and loudness hypersensitivity triggered by cochlear sensorineural damage. We found that a single 16-minute bout of 40Hz PVN stimulation session restored normal loudness perception for one week, showing that perceptual deficits triggered by irreversible peripheral injuries can be reversed through targeted cortical circuit interventions.
Keywords: Autism; aging; gamma stimulation; hallucination; hearing loss; hyperacusis; inhibition stabilized network; parvalbumin; schizophrenia; tinnitus.
Conflict of interest statement
Declaration of interests The authors have no competing interests to declare.
Figures






References
-
- Sachs M.B., and Abbas P.J. (1974). Rate versus level functions for auditory-nerve fibers in cats: tone-burst stimuli. 1847, 1835–1847. - PubMed
-
- Tobin M., Sheth J., Wood K.C., Michel E.K., and Geffen M.N. (2024). Differential modulation of cortical codes for sounds of varying intensity by distinct inhibitory neurons. bioRxiv. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
