This is a preprint.
Reduced Sialylation of Airway Mucin Impairs Mucus Transport by Altering the Biophysical Properties of Mucin
- PMID: 38853971
- PMCID: PMC11160914
- DOI: 10.21203/rs.3.rs-4421613/v1
Reduced Sialylation of Airway Mucin Impairs Mucus Transport by Altering the Biophysical Properties of Mucin
Update in
-
Reduced sialylation of airway mucin impairs mucus transport by altering the biophysical properties of mucin.Sci Rep. 2024 Jul 17;14(1):16568. doi: 10.1038/s41598-024-66510-2. Sci Rep. 2024. PMID: 39019950 Free PMC article.
Abstract
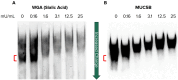
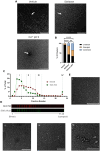
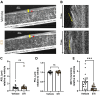
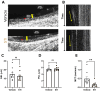
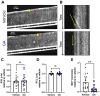
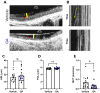
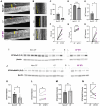
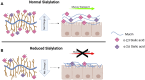
Mucus stasis is a pathologic hallmark of muco-obstructive diseases, including cystic fibrosis (CF). Mucins, the principal component of mucus, are extensively modified with hydroxyl (O)-linked glycans, which are largely terminated by sialic acid. Sialic acid is a negatively charged monosaccharide and contributes to the biochemical/biophysical properties of mucins. Reports suggest that mucin sialylation may be altered in CF; however, the consequences of reduced sialylation on mucus clearance have not been fully determined. Here, we investigated the consequences of reduced sialylation on the charge state and conformation of the most prominent airway mucin, MUC5B, and defined the functional consequences of reduced sialylation on mucociliary transport (MCT). Reduced sialylation contributed to a lower charged MUC5B form and decreased polymer expansion. The inhibition of total mucin sialylation de novo impaired MCT in primary human bronchial epithelial cells and rat airways, and specific α-2,3 sialylation blockade was sufficient to recapitulate these findings. Finally, we show that ST3 beta-galactoside alpha-2,3-sialyltransferase (ST3Gal1) expression is downregulated in CF and partially restored by correcting CFTR via Elexacaftor/Tezacaftor/Ivacaftor treatment. Overall, this study demonstrates the importance of mucin sialylation in mucus clearance and identifies decreased sialylation by ST3Gal1 as a possible therapeutic target in CF and potentially other muco-obstructive diseases.
Conflict of interest statement
Conflict of Interest: The authors have declared that no conflict of interest exists.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

