This is a preprint.
Psilocybin reduces heroin seeking behavior and modulates inflammatory gene expression in the nucleus accumbens and prefrontal cortex of male rats
- PMID: 38854027
- PMCID: PMC11160682
- DOI: 10.1101/2024.05.28.596205
Psilocybin reduces heroin seeking behavior and modulates inflammatory gene expression in the nucleus accumbens and prefrontal cortex of male rats
Update in
-
Psilocybin reduces heroin seeking behavior and modulates inflammatory gene expression in the nucleus accumbens and prefrontal cortex of male rats.Mol Psychiatry. 2025 May;30(5):1801-1816. doi: 10.1038/s41380-024-02788-y. Epub 2024 Oct 21. Mol Psychiatry. 2025. PMID: 39433903 Free PMC article.
Abstract
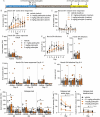
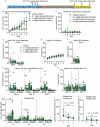
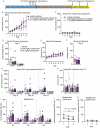
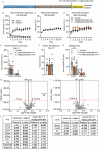
Preclinical and human studies indicate psilocybin may reduce perseverant maladaptive behaviors, including nicotine and alcohol seeking. Such studies in the opioid field are lacking, though opioids are involved in more >50% of overdose deaths. Psilocybin is an agonist at the serotonin 2A receptor (5-HT2AR), a well-documented target for modulation of drug seeking, and evidence suggests 5-HT2AR agonists may dampen motivation for opioids. We sought to investigate the therapeutic efficacy of psilocybin in mediating cessation of opioid use and maintenance of long-lasting abstinence from opioid seeking behavior in a rat model of heroin self-administration (SA). Psilocybin or 5-HT2AR antagonists ketanserin and volinanserin were administered systemically to rats prior to SA of 0.075 mg/kg/infusion of heroin, or relapse following forced abstinence. Psilocybin did not alter heroin taking, but a single exposure to 3.0 mg/kg psilocybin 4-24 hours prior to a relapse test blunted cue-induced heroin seeking. Conversely, 5-HT2AR antagonists exacerbated heroin relapse. To begin to elucidate mechanisms of psilocybin, drug-naïve rats received psilocybin and/or ketanserin, and tissue was collected from the prefrontal cortex (PFC), a region critical for drug seeking and responsive to psilocybin, 24 hours later for RNA-sequencing. 3.0 mg/kg psilocybin regulated ~2-fold more genes in the PFC than 1.0 mg/kg, including genes involved in the cytoskeleton and cytokine signaling. Ketanserin blocked >90% of psilocybin-regulated genes, including the IL-17a cytokine receptor, Il17ra. Psychedelic compounds have reported anti-inflammatory properties, and therefore we performed a gene expression array to measure chemokine/cytokine molecules in the PFC of animals that displayed psilocybin-mediated inhibition of heroin seeking. Psilocybin regulated 4 genes, including Il17a, and a subset of genes correlated with relapse behavior. Selective inhibition of PFC IL-17a was sufficient to reduce heroin relapse. We conclude that psilocybin reduces heroin relapse and highlight IL-17a signaling as a potential downstream pathway of psilocybin that also reduces heroin seeking.
Keywords: cortex; drug-seeking; heroin; inflammation; nucleus accumbens; opioid; psilocybin; psychedelic; relapse; self-administration; serotonin; transcriptome.
Conflict of interest statement
Competing Interests: We have no competing interests to disclose.
Figures

Similar articles
-
Psilocybin reduces heroin seeking behavior and modulates inflammatory gene expression in the nucleus accumbens and prefrontal cortex of male rats.Mol Psychiatry. 2025 May;30(5):1801-1816. doi: 10.1038/s41380-024-02788-y. Epub 2024 Oct 21. Mol Psychiatry. 2025. PMID: 39433903 Free PMC article.
-
Increased Excitability and Synaptic Plasticity of Drd1- and Drd2-Expressing Prelimbic Neurons Projecting to Nucleus Accumbens after Heroin Abstinence Are Reversed by Cue-Induced Relapse and Protein Kinase A Inhibition.J Neurosci. 2023 May 31;43(22):4019-4032. doi: 10.1523/JNEUROSCI.0108-23.2023. Epub 2023 Apr 24. J Neurosci. 2023. PMID: 37094933 Free PMC article.
-
Orbitofrontal cortex microRNAs support long-lasting heroin seeking behavior in male rats.Transl Psychiatry. 2023 Apr 8;13(1):117. doi: 10.1038/s41398-023-02423-4. Transl Psychiatry. 2023. PMID: 37031193 Free PMC article.
-
Dose titration with the glucagon-like peptide-1 agonist, liraglutide, reduces cue- and drug-induced heroin seeking in high drug-taking rats.Brain Res Bull. 2022 Oct 15;189:163-173. doi: 10.1016/j.brainresbull.2022.08.022. Epub 2022 Aug 28. Brain Res Bull. 2022. PMID: 36038016 Free PMC article. Review.
-
Acute treatment with the glucagon-like peptide-1 receptor agonist, liraglutide, reduces cue- and drug-induced fentanyl seeking in rats.Brain Res Bull. 2022 Oct 15;189:155-162. doi: 10.1016/j.brainresbull.2022.08.023. Epub 2022 Aug 27. Brain Res Bull. 2022. PMID: 36031011 Review.
References
-
- Hedegaard H, Miniño AM, Warner M. Co-involvement of opioids in drug overdose deaths involving cocaine and psychostimulants. NCHS Data Brief 2021; 406: 1–8. - PubMed
-
- Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa PC, Strassman RJ. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol 2015; 29(3): 289–299. - PubMed
-
- O’Donnell KC, Mennenga SE, Owens LT, Podrebarac SK, Baron T, Rotrosen J et al. Psilocybin for alcohol use disorder: Rationale and design considerations for a randomized controlled trial. Contemp Clin Trials 2022; 123: 106976. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
