This is a preprint.
Degree of Cyclooxygenase-2 Inhibition Modulates Blood Pressure Response to Celecoxib and Naproxen
- PMID: 38854091
- PMCID: PMC11160842
- DOI: 10.1101/2024.05.30.24308244
Degree of Cyclooxygenase-2 Inhibition Modulates Blood Pressure Response to Celecoxib and Naproxen
Update in
-
Degree of Cyclooxygenase-2 Inhibition Modulates Blood Pressure Response.Hypertension. 2026 Feb;83(2):e25516. doi: 10.1161/HYPERTENSIONAHA.124.25516. Epub 2025 Dec 29. Hypertension. 2026. PMID: 41459594 Free PMC article. Clinical Trial.
Abstract
Background: Non-steroidal anti-inflammatory drugs (NSAIDs) increase the risk of adverse cardiovascular events via suppression of cyclooxygenase (COX)-2-derived prostacyclin (PGI2) formation in heart, vasculature, and kidney. The Prospective Randomized Evaluation of Celecoxib Integrated Safety versus Ibuprofen Or Naproxen (PRECISION) trial and other large clinical studies compared the cardiovascular risk of traditional NSAIDs (i.e. naproxen), which inhibit both COX isozymes, with NSAIDs selective for COX-2 (i.e. celecoxib). However, whether pharmacologically equipotent doses were used - that is, whether a similar degree of COX-2 inhibition was achieved - was not considered. We compared drug target inhibition and blood pressure response to celecoxib at the dose used by most patients in PRECISION with the lowest recommended naproxen dose for osteoarthritis, which is lower than the dose used in PRECISION.
Methods: Sixteen healthy participants (19-61 years) were treated with celecoxib (100 mg every 12h), naproxen (250 mg every 12h), or placebo administered twice daily for seven days in a double-blind, crossover design randomized by order. On Day 7 when drug levels had reached steady state, the degree of COX inhibition was assessed ex vivo and in vivo. Ambulatory blood pressure was measured throughout the final 12h dosing interval.
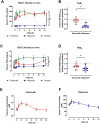
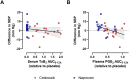
Results: Both NSAIDs inhibited COX-2 activity relative to placebo, but naproxen inhibited COX-2 activity to a greater degree (62.9±21.7%) than celecoxib (35.7±25.2%; p<0.05). Similarly, naproxen treatment inhibited PGI2 formation in vivo (48.0±24.9%) to a greater degree than celecoxib (26.7±24.6%; p<0.05). Naproxen significantly increased blood pressure compared to celecoxib (differences in least-square means of mean arterial pressure: 2.5 mm Hg (95% CI: 1.5, 3.5); systolic blood pressure: 4.0 mm Hg (95% CI: 2.9, 5.1); diastolic blood pressure: 1.8 mm Hg (95% CI: 0.8, 2.8); p<0.05 for all). The difference in systolic blood pressure relative to placebo was associated with the degree of COX-2 inhibition (p<0.05).
Conclusions: Celecoxib 200 mg/day inhibited COX-2 activity to a lesser degree than naproxen 500 mg/day, resulting in a less pronounced blood pressure increase. While the PRECISION trial concluded the non-inferiority of celecoxib regarding cardiovascular risk, this is based on a comparison of doses that are not equipotent.ClinicalTrials.gov identifier: NCT02502006 (https://clinicaltrials.gov/study/NCT02502006).
Figures

References
-
- In: Phillips JK, Ford MA, Bonnie RJ, eds. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington (DC); 2017. - PubMed
-
- Stagnitti MN. Trends in Outpatient Prescription Analgesics Utilization and Expenditures for the U.S. Civilian Noninstitutionalized Population, 1996 and 2006. Medical Expenditure Panel Survey. https://meps.ahrq.gov/data_files/publications/st235/stat235.shtml. 2009.Accessed 1/5/2024.
Publication types
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
