This is a preprint.
Dopamine-mediated plasticity preserves excitatory connections to direct pathway striatal projection neurons and motor function in a mouse model of Parkinson's disease
- PMID: 38854096
- PMCID: PMC11160626
- DOI: 10.1101/2024.05.28.596192
Dopamine-mediated plasticity preserves excitatory connections to direct pathway striatal projection neurons and motor function in a mouse model of Parkinson's disease
Abstract
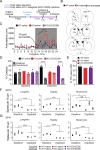
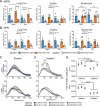
The cardinal symptoms of Parkinson's disease (PD) such as bradykinesia and akinesia are debilitating, and treatment options remain inadequate. The loss of nigrostriatal dopamine neurons in PD produces motor symptoms by shifting the balance of striatal output from the direct (go) to indirect (no-go) pathway in large part through changes in the excitatory connections and intrinsic excitabilities of the striatal projection neurons (SPNs). Here, we report using two different experimental models that a transient increase in striatal dopamine and enhanced D1 receptor activation, during 6-OHDA dopamine depletion, prevent the loss of mature spines and dendritic arbors on direct pathway projection neurons (dSPNs) and normal motor behavior for up to 5 months. The primary motor cortex and midline thalamic nuclei provide the major excitatory connections to SPNs. Using ChR2-assisted circuit mapping to measure inputs from motor cortex M1 to dorsolateral dSPNs, we observed a dramatic reduction in both experimental model mice and controls following dopamine depletion. Changes in the intrinsic excitabilities of SPNs were also similar to controls following dopamine depletion. Future work will examine thalamic connections to dSPNs. The findings reported here reveal previously unappreciated plasticity mechanisms within the basal ganglia that can be leveraged to treat the motor symptoms of PD.
Keywords: Direct Pathway; Dopamine; Motor Function; Parkinson’s disease; Plasticity; Spiny Projection Neuron; Striatum.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Similar articles
-
Pathway-specific dysregulation of striatal excitatory synapses by LRRK2 mutations.Elife. 2020 Oct 2;9:e58997. doi: 10.7554/eLife.58997. Elife. 2020. PMID: 33006315 Free PMC article.
-
Differential Synaptic Remodeling by Dopamine in Direct and Indirect Striatal Projection Neurons in Pitx3-/- Mice, a Genetic Model of Parkinson's Disease.J Neurosci. 2018 Apr 11;38(15):3619-3630. doi: 10.1523/JNEUROSCI.3184-17.2018. Epub 2018 Feb 26. J Neurosci. 2018. PMID: 29483281 Free PMC article.
-
A Population of Indirect Pathway Striatal Projection Neurons Is Selectively Entrained to Parkinsonian Beta Oscillations.J Neurosci. 2017 Oct 11;37(41):9977-9998. doi: 10.1523/JNEUROSCI.0658-17.2017. Epub 2017 Aug 28. J Neurosci. 2017. PMID: 28847810 Free PMC article.
-
Differential striatal spine pathology in Parkinson's disease and cocaine addiction: a key role of dopamine?Neuroscience. 2013 Oct 22;251:2-20. doi: 10.1016/j.neuroscience.2013.07.011. Epub 2013 Jul 16. Neuroscience. 2013. PMID: 23867772 Free PMC article. Review.
-
Loss and remodeling of striatal dendritic spines in Parkinson's disease: from homeostasis to maladaptive plasticity?J Neural Transm (Vienna). 2018 Mar;125(3):431-447. doi: 10.1007/s00702-017-1735-6. Epub 2017 May 24. J Neural Transm (Vienna). 2018. PMID: 28540422 Free PMC article. Review.
References
-
- Albin R.L., Young A.B., and Penney J.B. (1989). The functional anatomy of basal ganglia disorders. 12, 336–375. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
