This is a preprint.
Photoreceptor loss does not recruit neutrophils despite strong microglial activation
- PMID: 38854151
- PMCID: PMC11160676
- DOI: 10.1101/2024.05.25.595864
Photoreceptor loss does not recruit neutrophils despite strong microglial activation
Update in
-
Photoreceptor loss does not recruit neutrophils despite strong microglial activation.Elife. 2025 Jul 22;13:RP98662. doi: 10.7554/eLife.98662. Elife. 2025. PMID: 40694413 Free PMC article.
Abstract
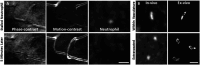
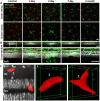
In response to central nervous system (CNS) injury, tissue resident immune cells such as microglia and circulating systemic neutrophils are often first responders. The degree to which these cells interact in response to CNS damage is poorly understood, and even less so, in the neural retina which poses a challenge for high resolution imaging in vivo. In this study, we deploy fluorescence adaptive optics scanning light ophthalmoscopy (AOSLO) to study microglia and neutrophils in mice. We simultaneously track immune cell dynamics using label-free phase-contrast AOSLO at micron-level resolution. Retinal lesions were induced with 488 nm light focused onto photoreceptor (PR) outer segments. These lesions focally ablated PRs, with minimal collateral damage to cells above and below the plane of focus. We used in vivo (AOSLO, SLO and OCT) imaging to reveal the natural history of the microglial and neutrophil response from minutes-to-months after injury. While microglia showed dynamic and progressive immune response with cells migrating into the injury locus within 1-day after injury, neutrophils were not recruited despite close proximity to vessels carrying neutrophils only microns away. Post-mortem confocal microscopy confirmed in vivo findings. This work illustrates that microglial activation does not recruit neutrophils in response to acute, focal loss of PRs, a condition encountered in many retinal diseases.
Conflict of interest statement
Portions of this work were funded by a collaborative grant from Genentech, Inc. (Elstrott) to examine the extent to which immune cells could be studied in the deep retina using adaptive optics. Schallek also has six patents held through the University of Rochester on adaptive optics technology.
Figures
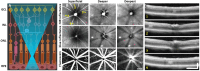
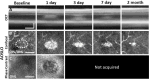


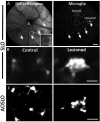
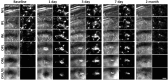


References
-
- Stone J. et al. Mechanisms of photoreceptor death and survival in mammalian retina. Progress in Retinal and Eye Research 18, 689–735 (1999). - PubMed
-
- Boycott B. B. & Hopkins J. M. Microglia in the retina of monkey and other mammals: its distinction from other types of glia and horizontal cells. Neuroscience 6, 679–688 (1981). - PubMed
-
- Silverman S. M. & Wong W. T. Microglia in the Retina: Roles in Development, Maturity, and Disease. Annual Review of Vision Science 4, 45–77 (2018). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
