This is a preprint.
Benzylic Trifluoromethyl Accelerates 1,6-Elimination Toward Rapid Probe Activation
- PMID: 38854154
- PMCID: PMC11160802
- DOI: 10.1101/2024.05.30.596105
Benzylic Trifluoromethyl Accelerates 1,6-Elimination Toward Rapid Probe Activation
Abstract
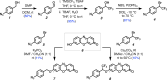
Activity-based detection of hydrogen sulfide in live cells can expand our understanding of its reactivity and complex physiological effects. We have discovered a highly efficient method for fluorescent probe activation, which is driven by H2S-triggered 1,6-elimination of an α-CF3-benzyl to release resorufin. In detecting intracellular H2S, 4-azido-(α-CF3)-benzyl resorufin offers significantly faster signal generation and improved sensitivity compared to 4-azidobenzyl resorufin. Computed free energy profiles for the 1,6-elimination process support the hypothesis that a benzylic CF3 group can reduce the activation energy barrier toward probe activation. This novel probe design allows for near-real-time detection of H2S in HeLa cells under stimulation conditions.
Conflict of interest statement
DECLARATION OF INTEREST The authors declare no competing interests.
Figures





Similar articles
-
Hydrogen sulfide detection and zebrafish imaging by a designed sensitive and selective fluorescent probe based on resorufin.Spectrochim Acta A Mol Biomol Spectrosc. 2022 Jan 5;264:120265. doi: 10.1016/j.saa.2021.120265. Epub 2021 Aug 6. Spectrochim Acta A Mol Biomol Spectrosc. 2022. PMID: 34455378
-
A highly sensitive endoplasmic reticulum-targeting fluorescent probe for the imaging of endogenous H2S in live cells.Spectrochim Acta A Mol Biomol Spectrosc. 2020 Oct 15;240:118578. doi: 10.1016/j.saa.2020.118578. Epub 2020 Jun 5. Spectrochim Acta A Mol Biomol Spectrosc. 2020. PMID: 32534426
-
Mitochondrion-targeting near-infrared fluorescent probe for detecting intracellular nanomolar level hydrogen sulfide with high recognition rate.Anal Bioanal Chem. 2021 Feb;413(4):1215-1224. doi: 10.1007/s00216-020-03086-6. Epub 2021 Jan 2. Anal Bioanal Chem. 2021. PMID: 33386936
-
A unique off-on near-infrared QCy7-derived probe for selective detection and imaging of hydrogen sulfide in cells and in vivo.Spectrochim Acta A Mol Biomol Spectrosc. 2020 Feb 5;226:117635. doi: 10.1016/j.saa.2019.117635. Epub 2019 Oct 8. Spectrochim Acta A Mol Biomol Spectrosc. 2020. PMID: 31605973
-
A 3,5-dinitropyridin-2yl Substituted Flavonol-based Fluorescent Probe for Rapid Detection of H2S in Water, Foodstuff Samples and Living Cells.J Fluoresc. 2024 Sep;34(5):1945-1954. doi: 10.1007/s10895-023-03427-5. Epub 2023 Sep 6. J Fluoresc. 2024. PMID: 37672181 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
