Outcomes and Predictors of Mortality in Patients With KPC-Kp Infections Treated With Meropenem Vaborbactam: An Observational Multicenter Study
- PMID: 38854388
- PMCID: PMC11161898
- DOI: 10.1093/ofid/ofae273
Outcomes and Predictors of Mortality in Patients With KPC-Kp Infections Treated With Meropenem Vaborbactam: An Observational Multicenter Study
Abstract
Background: Meropenem-vaborbactam is a recent and promising option for the treatment of KPC-producing Klebsiella pneumoniae (KPC-Kp) infections, including those resistant to ceftazidime-avibactam.
Methods: We conducted a retrospective analysis of observational data from 19 Italian hospitals on use and outcomes of patients treated with meropenem-vaborbactam for at least ≥24 hours for KPC-Kp infections. Crude and propensity-weighted multiple Cox regression models were performed to ascertain risk factors independently associated with 30-day mortality.
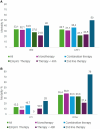
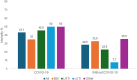
Results: The cohort included 342 adults with bloodstream infections (n = 172) and nonbacteremic infections (n = 170), of which 107 were lower respiratory tract infections, 30 were complicated urinary tract infections, and 33 were infections involving other sites. Most infections (62.3%) were managed with meropenem-vaborbactam monotherapy, or in combination with at least 1 other active drug (usually fosfomycin, tigecycline, or gentamicin) (37.7%). The 30-day mortality rate was 31.6% (108/342). In multiple Cox regression model, 30-day mortality was independently associated with septic shock at infection onset, Charlson comorbidity index ≥ 3, dialysis, concomitant COVID-19, and INCREMENT score ≥ 8. Administration of meropenem-vaborbactam within 48 hours from infection onset was a negative predictor of mortality. All predictors, except administration of meropenem-vaborbactam within 48 hours, remained significant when the multiple Cox regression model was repeated after adjustment for the propensity score for receipt of combination therapy.
Conclusions: Despite the limits of a retrospective study, the data derived from this multicenter cohort provide additional evidence on the efficacy of meropenem-vaborbactam in treating severe KPC-Kp infections, even when used as monotherapy.
Keywords: KPC-producing Klebsiella pneumoniae; bloodstream infection; carbapenemases; ceftazidime-avibactam resistance; meropenem-vaborbactam.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. No reported conflicts of interest
Figures
References
-
- Tumbarello M, Viale P, Bassetti M, et al. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study—authors’ response. J Antimicrob Chemother 2015; 70:2922. - PubMed
-
- Bassetti M, Giacobbe DR, Giamarellou H, et al. Management of KPC-producing Klebsiella pneumoniae infections. Clin Microbiol Infect 2018; 24:133–44. - PubMed
-
- Di Bella S, Giacobbe DR, Maraolo AE, et al. Resistance to ceftazidime/avibactam in infections and colonisations by KPC-producing Enterobacterales: a systematic review of observational clinical studies. J Glob Antimicrob Resist 2021; 25:268–81. - PubMed

