Monitoring mitochondrial calcium in cardiomyocytes during coverslip hypoxia using a fluorescent lifetime indicator
- PMID: 38854449
- PMCID: PMC11156168
- DOI: 10.1016/j.jmccpl.2024.100074
Monitoring mitochondrial calcium in cardiomyocytes during coverslip hypoxia using a fluorescent lifetime indicator
Abstract
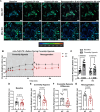
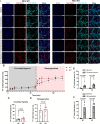
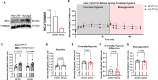
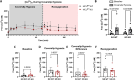
An increase in mitochondrial calcium via the mitochondrial calcium uniporter (MCU) has been implicated in initiating cell death in the heart during ischemia-reperfusion (I/R) injury. Measurement of calcium during I/R has been challenging due to the pH sensitivity of indicators coupled with the fall in pH during I/R. The development of a pH-insensitive indicator, mitochondrial localized Turquoise Calcium fluorescence Lifetime Sensor (mito-TqFLITS), allows for quantifying mitochondrial calcium during I/R via fluorescent lifetime imaging. Mitochondrial calcium was monitored using mito-TqFLITS, in neonatal mouse ventricular myocytes (NMVM) isolated from germline MCU-KO mice and MCUfl/fl treated with CRE-recombinase to acutely knockout MCU. To simulate ischemia, a coverslip was placed on a monolayer of NMVMs to prevent access to oxygen and nutrients. Reperfusion was induced by removing the coverslip. Mitochondrial calcium increases threefold during coverslip hypoxia in MCU-WT. There is a significant increase in mitochondrial calcium during coverslip hypoxia in germline MCU-KO, but it is significantly lower than in MCU-WT. We also found that compared to WT, acute MCU-KO resulted in no difference in mitochondrial calcium during coverslip hypoxia and reoxygenation. To determine the role of mitochondrial calcium uptake via MCU in initiating cell death, we used propidium iodide to measure cell death. We found a significant increase in cell death in both the germline MCU-KO and acute MCU-KO, but this was similar to their respective WTs. These data demonstrate the utility of mito-TqFLITS to monitor mitochondrial calcium during simulated I/R and further show that germline loss of MCU attenuates the rise in mitochondrial calcium during ischemia but does not reduce cell death.
Keywords: calcium; cardioprotection; cell death; fluorescent lifetime imaging; ischemia-reperfusion; mitochondria.
Conflict of interest statement
Declaration of interests The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Increased mitochondrial free Ca2+ during ischemia is suppressed, but not eliminated by, germline deletion of the mitochondrial Ca2+ uniporter.Cell Rep. 2023 Jul 25;42(7):112735. doi: 10.1016/j.celrep.2023.112735. Epub 2023 Jul 7. Cell Rep. 2023. PMID: 37421627 Free PMC article.
-
Mitochondrial membrane potential instability on reperfusion after ischemia does not depend on mitochondrial Ca2+ uptake.J Biol Chem. 2023 Jun;299(6):104708. doi: 10.1016/j.jbc.2023.104708. Epub 2023 Apr 14. J Biol Chem. 2023. PMID: 37061004 Free PMC article.
-
Cyclophilin D-mediated regulation of the permeability transition pore is altered in mice lacking the mitochondrial calcium uniporter.Cardiovasc Res. 2019 Feb 1;115(2):385-394. doi: 10.1093/cvr/cvy218. Cardiovasc Res. 2019. PMID: 30165576 Free PMC article.
-
The debate continues - What is the role of MCU and mitochondrial calcium uptake in the heart?J Mol Cell Cardiol. 2020 Jun;143:163-174. doi: 10.1016/j.yjmcc.2020.04.029. Epub 2020 Apr 27. J Mol Cell Cardiol. 2020. PMID: 32353353 Free PMC article. Review.
-
The mitochondrial calcium uniporter: mice can live and die without it.J Mol Cell Cardiol. 2015 Jan;78:46-53. doi: 10.1016/j.yjmcc.2014.10.013. Epub 2014 Nov 4. J Mol Cell Cardiol. 2015. PMID: 25451167 Free PMC article. Review.
Cited by
-
How does mitochondrial Ca2+ change during ischemia and reperfusion? Implications for activation of the permeability transition pore.J Gen Physiol. 2025 Jan 6;157(1):e202313520. doi: 10.1085/jgp.202313520. Epub 2024 Dec 19. J Gen Physiol. 2025. PMID: 39699565 Review.
-
Mechanisms of postischemic cardiac death and protection following myocardial injury.J Clin Invest. 2025 Jan 2;135(1):e184134. doi: 10.1172/JCI184134. J Clin Invest. 2025. PMID: 39744953 Free PMC article. Review.
References
-
- Murphy E., Perlman M., London R.E., Steenbergen C. Amiloride delays the ischemia-induced rise in cytosolic free calcium. Circ Res. 1991;68(5):1250–1258. - PubMed
-
- Steenbergen C., Murphy E., Levy L., London R.E. Elevation in cytosolic free calcium-concentration early in myocardial-ischemia in perfused rat-heart. Circ Res. 1987;60(5):700–707. - PubMed
-
- Garcia-Dorado D., Ruiz-Meana M., Inserte J., Rodriguez-Sinovas A., Piper H.M. Calcium-mediated cell death during myocardial reperfusion. Cardiovasc Res. 2012;94(2):168–180. - PubMed
-
- Halestrap A.P. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem. Soc. T. 2006;34:232–237. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

