Manipulating calcium homeostasis with nanoplatforms for enhanced cancer therapy
- PMID: 38854493
- PMCID: PMC10867402
- DOI: 10.1002/EXP.20230019
Manipulating calcium homeostasis with nanoplatforms for enhanced cancer therapy
Abstract
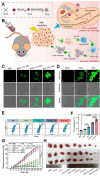
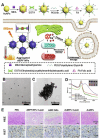
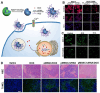
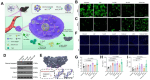
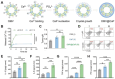
Calcium ions (Ca2+) are indispensable and versatile metal ions that play a pivotal role in regulating cell metabolism, encompassing cell survival, proliferation, migration, and gene expression. Aberrant Ca2+ levels are frequently linked to cell dysfunction and a variety of pathological conditions. Therefore, it is essential to maintain Ca2+ homeostasis to coordinate body function. Disrupting the balance of Ca2+ levels has emerged as a potential therapeutic strategy for various diseases, and there has been extensive research on integrating this approach into nanoplatforms. In this review, the current nanoplatforms that regulate Ca2+ homeostasis for cancer therapy are first discussed, including both direct and indirect approaches to manage Ca2+ overload or inhibit Ca2+ signalling. Then, the applications of these nanoplatforms in targeting different cells to regulate their Ca2+ homeostasis for achieving therapeutic effects in cancer treatment are systematically introduced, including tumour cells and immune cells. Finally, perspectives on the further development of nanoplatforms for regulating Ca2+ homeostasis, identifying scientific limitations and future directions for exploitation are offered.
Keywords: Ca2+ inhibition; Ca2+ overload; calcium homeostasis regulation; cancer therapy; immunotherapy.
© 2023 The Authors. Exploration published by Henan University and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures













References
-
- Berridge M. J., Lipp P., Bootman M. D., Nat. Rev. Mol. Cell Biol. 2000, 1, 11. - PubMed
-
- Clapham D. E., Cell 2007, 131, 1047. - PubMed
-
- Berridge M. J., Physiol. Rev. 2016, 96, 1261. - PubMed
-
- De Stefani D., Rizzuto R., Pozzan T., Annu. Rev. Biochem. 2016, 85, 161. - PubMed
-
- Giorgi C., Danese A., Missiroli S., Patergnani S., Pinton P., Trends Cell Biol. 2018, 28, 258. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
