Emerging strategies for combating Fusobacterium nucleatum in colorectal cancer treatment: Systematic review, improvements and future challenges
- PMID: 38854496
- PMCID: PMC10867388
- DOI: 10.1002/EXP.20230092
Emerging strategies for combating Fusobacterium nucleatum in colorectal cancer treatment: Systematic review, improvements and future challenges
Abstract
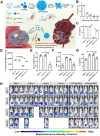
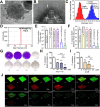
Colorectal cancer (CRC) is generally characterized by a high prevalence of Fusobacterium nucleatum (F. nucleatum), a spindle-shaped, Gram-negative anaerobe pathogen derived from the oral cavity. This tumor-resident microorganism has been closely correlated with the occurrence, progression, chemoresistance and immunosuppressive microenvironment of CRC. Furthermore, F. nucleatum can specifically colonize CRC tissues through adhesion on its surface, forming biofilms that are highly resistant to commonly used antibiotics. Accordingly, it is crucial to develop efficacious non-antibiotic approaches to eradicate F. nucleatum and its biofilms for CRC treatment. In recent years, various antimicrobial strategies, such as natural extracts, inorganic chemicals, organic chemicals, polymers, inorganic-organic hybrid materials, bacteriophages, probiotics, and vaccines, have been proposed to combat F. nucleatum and F. nucleatum biofilms. This review summarizes the latest advancements in anti-F. nucleatum research, elucidates the antimicrobial mechanisms employed by these systems, and discusses the benefits and drawbacks of each antimicrobial technology. Additionally, this review also provides an outlook on the antimicrobial specificity, potential clinical implications, challenges, and future improvements of these antimicrobial strategies in the treatment of CRC.
Keywords: Fusobacterium nucleatum; antibacterial; anti‐biofilm; colorectal cancer; drug resistance.
© 2023 The Authors. Exploration published by Henan University and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
The role of Fusobacterium nucleatum in colorectal cancer: from carcinogenesis to clinical management.Chronic Dis Transl Med. 2019 Oct 1;5(3):178-187. doi: 10.1016/j.cdtm.2019.09.001. eCollection 2019 Sep. Chronic Dis Transl Med. 2019. PMID: 31891129 Free PMC article.
-
Fusobacterium nucleatum Acts as a Pro-carcinogenic Bacterium in Colorectal Cancer: From Association to Causality.Front Cell Dev Biol. 2021 Aug 20;9:710165. doi: 10.3389/fcell.2021.710165. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34490259 Free PMC article. Review.
-
A Novel Bacteriophage with the Potential to Inhibit Fusobacterium nucleatum-Induced Proliferation of Colorectal Cancer Cells.Antibiotics (Basel). 2025 Jan 7;14(1):45. doi: 10.3390/antibiotics14010045. Antibiotics (Basel). 2025. PMID: 39858331 Free PMC article.
-
Proteomic characterization of outer membrane vesicles from gut mucosa-derived fusobacterium nucleatum.J Proteomics. 2019 Mar 20;195:125-137. doi: 10.1016/j.jprot.2018.12.029. Epub 2019 Jan 8. J Proteomics. 2019. PMID: 30634002
-
Fusobacterium nucleatum, a key pathogenic factor and microbial biomarker for colorectal cancer.Trends Microbiol. 2023 Feb;31(2):159-172. doi: 10.1016/j.tim.2022.08.010. Epub 2022 Sep 1. Trends Microbiol. 2023. PMID: 36058786 Review.
Cited by
-
Preclinical Assessment of Living Therapeutic Materials: State-of-Art and Challenges.ACS Biomater Sci Eng. 2025 May 12;11(5):2584-2600. doi: 10.1021/acsbiomaterials.5c00247. Epub 2025 Apr 15. ACS Biomater Sci Eng. 2025. PMID: 40230223 Free PMC article. Review.
-
Various Antibacterial Strategies Utilizing Titanium Dioxide Nanotubes Prepared via Electrochemical Anodization Biofabrication Method.Biomimetics (Basel). 2024 Jul 5;9(7):408. doi: 10.3390/biomimetics9070408. Biomimetics (Basel). 2024. PMID: 39056849 Free PMC article. Review.
-
Unveiling the intratumoral microbiota within cancer landscapes.iScience. 2024 May 3;27(6):109893. doi: 10.1016/j.isci.2024.109893. eCollection 2024 Jun 21. iScience. 2024. PMID: 38799560 Free PMC article. Review.
-
Recent advances in therapeutic probiotics: insights from human trials.Clin Microbiol Rev. 2025 Jun 12;38(2):e0024024. doi: 10.1128/cmr.00240-24. Epub 2025 Apr 22. Clin Microbiol Rev. 2025. PMID: 40261032 Review.
-
The gut microbiota in cancer immunity and immunotherapy.Cell Mol Immunol. 2025 Sep;22(9):1012-1031. doi: 10.1038/s41423-025-01326-2. Epub 2025 Aug 6. Cell Mol Immunol. 2025. PMID: 40770084 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
