Highly Stable Amorphous (Pyro)phosphate Aggregates: Pyrophosphate as a Carrier for Bioactive Ions and Drugs in Bone Repair Applications
- PMID: 38854518
- PMCID: PMC11154929
- DOI: 10.1021/acsomega.4c01660
Highly Stable Amorphous (Pyro)phosphate Aggregates: Pyrophosphate as a Carrier for Bioactive Ions and Drugs in Bone Repair Applications
Abstract
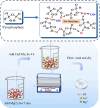
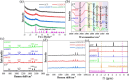
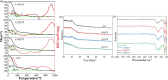
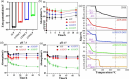
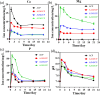
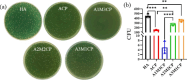
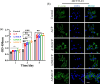
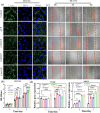
Pyrophosphate is widely used as an iron supplement because of its excellent complexation and hydrolysis ability; however, there are few reports on the use of pyrophosphate in active ionophores for bone repair. In this research, we proposed a simple and efficient ultrasonic method to prepare magnesium-calcium (pyro)phosphate aggregates (AMCPs). Due to strong hydration, AMCPs maintain a stable amorphous form even at high temperatures (400 °C). By changing the molar ratio of calcium and magnesium ions, the content of calcium and magnesium ions can be customized. AMCPs had surface negativity and complexing ability that realized the controlled release of ions (Ca2+, Mg2+, and P) and drugs (such as doxorubicin) over a long period. Pyrophosphate gave it an excellent bacteriostatic effect. Increasingly released Mg2+ exhibited improved bioactivity though the content of Ca2+ decreased. While Mg2+ content was regulated to 15 wt %, it performed significantly enhanced stimulation on the proliferation, attachment, and differentiation (ALP activity, calcium nodules, and the related gene expression of osteogenesis) of mouse embryo osteoblast precursor cells (MC3T3-E1). Furthermore, the high content of Mg2+ also effectively promoted the proliferation, attachment, and migration of human umbilical vein endothelial cells (HUVECs) and the expression of angiogenic genes. In conclusion, pyrophosphate was an excellent carrier for bioactive ions, and the AMCPs we prepared had a variety of active functions for multiscenario bone repair applications.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Tunable Behavior in Solution of Amorphous Calcium Ortho/Pyrophosphate Materials: An Acellular In Vitro Study.ACS Biomater Sci Eng. 2022 Jun 13;8(6):2363-2374. doi: 10.1021/acsbiomaterials.1c01618. Epub 2022 May 9. ACS Biomater Sci Eng. 2022. PMID: 35533345
-
Development of a new family of monolithic calcium (pyro)phosphate glasses by soft chemistry.Acta Biomater. 2016 Sep 1;41:320-7. doi: 10.1016/j.actbio.2016.05.030. Epub 2016 May 21. Acta Biomater. 2016. PMID: 27221792
-
Exploring the interplay of mucin with biologically-relevant amorphous magnesium-calcium phosphate nanoparticles.J Colloid Interface Sci. 2021 Jul 15;594:802-811. doi: 10.1016/j.jcis.2021.03.062. Epub 2021 Mar 16. J Colloid Interface Sci. 2021. PMID: 33794402
-
Novel Co-akermanite (Ca2CoSi2O7) bioceramics with the activity to stimulate osteogenesis and angiogenesis.J Mater Chem B. 2015 Sep 7;3(33):6773-6782. doi: 10.1039/c5tb01244a. Epub 2015 Jul 29. J Mater Chem B. 2015. PMID: 32262470
-
Osteoblast response to zirconia-hybridized pyrophosphate-stabilized amorphous calcium phosphate.J Biomed Mater Res A. 2006 Mar 1;76(3):596-604. doi: 10.1002/jbm.a.30573. J Biomed Mater Res A. 2006. PMID: 16278876 Free PMC article.
Cited by
-
Alteration of Sulfur-Bearing Silicate-Phosphate (Agri)Glasses in Soil Environment: Structural Characterization and Chemical Reactivity of Fertilizer Glasses: Insights from 'In Vitro' Studies.Molecules. 2025 Apr 9;30(8):1684. doi: 10.3390/molecules30081684. Molecules. 2025. PMID: 40333601 Free PMC article.
References
-
- Dong Q.; Li Y.; Jiang H.; Zhou X.; Liu H.; Lu M.; Chu C.; Xue F.; Bai J. 3D-cubic interconnected porous Mg-based scaffolds for bone repair. J. Magnes. Alloy. 2021, 9 (4), 1329–1338. 10.1016/j.jma.2020.05.022. - DOI
-
- Maleki-Ghaleh H.; Siadati M.H.; Fallah A.; Koc B.; Kavanlouei M.; Khademi-Azandehi P.; Moradpur-Tari E.; Omidi Y.; Barar J.; Beygi-Khosrowshahi Y.; Kumar A.P.; Adibkia K. Antibacterial and Cellular Behaviors of Novel Zinc-Doped Hydroxyapatite/Graphene Nanocomposite for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22 (17), 9564.10.3390/ijms22179564. - DOI - PMC - PubMed
-
- Valletregi M. Calcium phosphates as substitution of bone tissues. Prog. Solid. State Ch. 2004, 32 (1–2), 1–31. 10.1016/j.progsolidstchem.2004.07.001. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
