Comparative Analysis of Primary and Secondary Metabolites in Different In Vitro Tissues of Narcissus tazetta var. chinensis
- PMID: 38854557
- PMCID: PMC11154942
- DOI: 10.1021/acsomega.4c01735
Comparative Analysis of Primary and Secondary Metabolites in Different In Vitro Tissues of Narcissus tazetta var. chinensis
Abstract
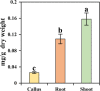
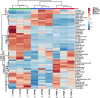
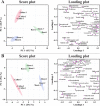
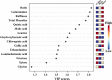
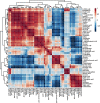
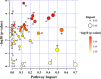
Narcissus tazetta var. chinensis is a perennial monocot plant that is well known for its pharmaceutical and ornamental uses. This study aimed to understand the changes in the primary and secondary metabolites in different in vitro tissues of N. tazetta (callus, adventitious root, and shoot) using high-performance liquid chromatography and gas chromatography time-of-flight mass spectrometry. In addition, to optimize the most efficient in vitro culture methods for primary and secondary metabolite production, N. tazetta bulbs were used as explants and cultivated in Murashige and Skoog (MS) medium containing different hormones at various concentrations. In addition, the present study found suitable hormonal concentrations for callus, adventitious root, and shoot induction and analyzed the primary and secondary metabolites. The MS medium supplemented with 1.0 mg L-1 dicamba, 3.0 mg L-1 indole-3-butyric acid (IBA), and 3.0 mg L-1 6-benzylaminopurine (BAP) was the most efficient media for callus, adventitious root, and shoot induction in N. tazetta. The tissue induced in this medium was subjected to primary (amines, amino acids, organic acids, sugars, and sugar alcohols) and secondary metabolite (galantamine and phenolic acids) analysis. The shoots and roots showed the highest amounts of metabolites. This study showed that bulb in vitro culture can be an efficient micropropagation method for N. tazetta and the production of primary and secondary metabolites, offering implications for the mass production of primary and secondary metabolite compounds from N. tazetta tissues generated in vitro.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Abdel-Rahman H.; Al-Ansary A.; Rashed K.; Rizkalla A. Micropropagation of Narcissus tazetta ‘Chinensis’ and its relation to secondary metabolites content. J. Appl. Life Sci. Int. 2017, 14 (1), 1–11. 10.9734/JALSI/2017/36410. - DOI
-
- Girard M.-P.; Karimzadegan V.; Héneault M.; Cloutier F.; Bérubé G.; Berthoux L.; Mérindol N.; Desgagné-Penix I. Chemical synthesis and biological activities of Amaryllidaceae alkaloid norbelladine derivatives and precursors. Molecules 2022, 27 (17), 5621.10.3390/molecules27175621. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
