General Characterization of Properties of Ordered and Disordered Proteins by Wide-Line 1H NMR
- PMID: 38854569
- PMCID: PMC11154930
- DOI: 10.1021/acsomega.4c00517
General Characterization of Properties of Ordered and Disordered Proteins by Wide-Line 1H NMR
Abstract
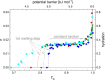
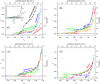
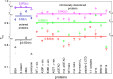
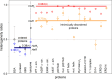
Wide-line 1H NMR is an efficient spectroscopic method to determine the disorder tendency of a protein. It directly measures the properties of the hydration shell of proteins, delivering exact and measurable values of their disorder/order content. A comparison is performed between several globular and disordered proteins. The common properties of the subzero mobile hydration water of these two groups were investigated. The amount of the mobile hydration water and the shape of the melting diagram at subzero temperatures together provide a possibility to distinguish globular proteins from disordered proteins. The shape of the melting diagram also gives information about the presence of secondary structural elements. The disordered and globular protein regions' fundamentally different structures are reflected in their melting diagrams, allowing one to directly determine the level of disorder in a specific protein structure. Intrinsically disordered proteins bind water more strongly than globular proteins, which is shown by the somewhat higher temperature values where mobile hydration water first appears but with a significantly higher heterogeneity in the energy distributions of protein-water interactions.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Tompa K.; Bokor M.; Verebélyi T.; Tompa P. Water rotation barriers on protein molecular surfaces. Chem. Phys. 2015, 448, 15–25. 10.1016/j.chemphys.2014.12.008. - DOI
-
- CRC Handbook of Chemistry and Physics, 103rd ed.; Rumble J., Ed.; CRC Press, 2022–2023. 9781032121710.
LinkOut - more resources
Full Text Sources
