Thermodynamic Reversal and Structural Correlation of 24-Crown-8/Protonated Tryptophan and 24-Crown 8/Protonated Serine Noncovalent Complexes in the Gas Phase vs in Solution: Quantum Chemical Analysis
- PMID: 38854571
- PMCID: PMC11154897
- DOI: 10.1021/acsomega.4c01782
Thermodynamic Reversal and Structural Correlation of 24-Crown-8/Protonated Tryptophan and 24-Crown 8/Protonated Serine Noncovalent Complexes in the Gas Phase vs in Solution: Quantum Chemical Analysis
Abstract
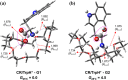
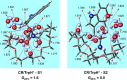
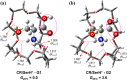
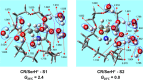
We investigate the structures of 24-crown-8/H+/l-tryptophan (CR/TrpH+) and 24-crown-8/H+/l-serine (CR/SerH+) noncovalent host-guest complex both in the gas phase and in an aqueous solution by quantum chemical methods. The Gibbs free energies of the complex in the two phases are calculated to determine the thermodynamically most favorable conformer in each phase. Our predictions indicate that both the carboxyl and the ammonium in CR/TrpH+ and the ammonium in the CR/SerH+ complexes in the lowest Gibbs free energy configurations form hydrogen bonds (H-bonds) with the CR host in the gas phase, while the conformer with the "naked" (devoid of H-bond with the CR host) -CO2H (and/or -OH) is much less favorable (Gibbs free energy higher by >3.6 kcal/mol). In the solution phase, however, a "thermodynamic reversal" occurs, making the higher Gibbs free energy gas-phase CR/TrpH+ and CR/SerH+ conformers thermodynamically more favorable under the influence of solvent molecules. Consequently, the global minimum Gibbs free energy structure in solution is structurally correlated with the thermodynamically much less gas-phase conformer. Discussions are provided concerning the possibility of elucidating host-guest-solvent interactions in solution from the gas-phase host-guest configurations in molecular detail.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
LinkOut - more resources
Full Text Sources
