Automated quantification of photoreceptor outer segments in developing and degenerating retinas on microscopy images across scales
- PMID: 38854587
- PMCID: PMC11157083
- DOI: 10.3389/fnmol.2024.1398447
Automated quantification of photoreceptor outer segments in developing and degenerating retinas on microscopy images across scales
Abstract
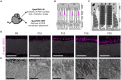
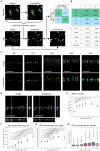
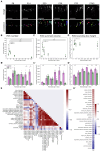
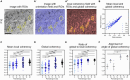
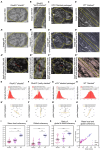
The functionality of photoreceptors, rods, and cones is highly dependent on their outer segments (POS), a cellular compartment containing highly organized membranous structures that generate biochemical signals from incident light. While POS formation and degeneration are qualitatively assessed on microscopy images, reliable methodology for quantitative analyses is still limited. Here, we developed methods to quantify POS (QuaPOS) maturation and quality on retinal sections using automated image analyses. POS formation was examined during the development and in adulthood of wild-type mice via light microscopy (LM) and transmission electron microscopy (TEM). To quantify the number, size, shape, and fluorescence intensity of POS, retinal cryosections were immunostained for the cone POS marker S-opsin. Fluorescence images were used to train the robust classifier QuaPOS-LM based on supervised machine learning for automated image segmentation. Characteristic features of segmentation results were extracted to quantify the maturation of cone POS. Subsequently, this quantification method was applied to characterize POS degeneration in "cone photoreceptor function loss 1" mice. TEM images were used to establish the ultrastructural quantification method QuaPOS-TEM for the alignment of POS membranes. Images were analyzed using a custom-written MATLAB code to extract the orientation of membranes from the image gradient and their alignment (coherency). This analysis was used to quantify the POS morphology of wild-type and two inherited retinal degeneration ("retinal degeneration 19" and "rhodopsin knock-out") mouse lines. Both automated analysis technologies provided robust characterization and quantification of POS based on LM or TEM images. Automated image segmentation by the classifier QuaPOS-LM and analysis of the orientation of membrane stacks by QuaPOS-TEM using fluorescent or TEM images allowed quantitative evaluation of POS formation and quality. The assessments showed an increase in POS number, volume, and membrane coherency during wild-type postnatal development, while a decrease in all three observables was detected in different retinal degeneration mouse models. All the code used for the presented analysis is open source, including example datasets to reproduce the findings. Hence, the QuaPOS quantification methods are useful for in-depth characterization of POS on retinal sections in developmental studies, for disease modeling, or after therapeutic interventions affecting photoreceptors.
Keywords: cone; electron microscopy; fluorescence microscopy; photoreceptor outer segment; retinal degeneration; retinal development; segmentation; supervised machine learning.
Copyright © 2024 Seidemann, Salomon, Hoffmann, Kurth, Sbalzarini, Haase and Ader.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Aguirre G. D., Cideciyan A. V., Dufour V. L., Ripolles-García A., Sudharsan R., Swider M., et al. (2021). Gene therapy reforms photoreceptor structure and restores vision in NPHP5-associated Leber congenital Amaurosis. Mol. Ther. 29, 2456–2468. doi: 10.1016/j.ymthe.2021.03.021, PMID: - DOI - PMC - PubMed
-
- Almedawar S., Vafia K., Schreiter S., Neumann K., Khattak S., Kurth T., et al. (2020). MERTK-dependent Ensheathment of photoreceptor outer segments by human pluripotent stem cell-derived retinal pigment epithelium. Stem Cell Reports 14, 374–389. doi: 10.1016/j.stemcr.2020.02.004, PMID: - DOI - PMC - PubMed
-
- Assawachananont J., Mandai M., Okamoto S., Yamada C., Eiraku M., Yonemura S., et al. (2014). Transplantation of embryonic and induced pluripotent stem cell-derived 3D retinal sheets into retinal degenerative mice. Stem Cell Reports 2, 662–674. doi: 10.1016/j.stemcr.2014.03.011, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

