Different narcotic gases and concentrations for immobilization of ostrich embryos for in-ovo imaging
- PMID: 38854792
- PMCID: PMC11157058
- DOI: 10.3389/ebm.2024.10037
Different narcotic gases and concentrations for immobilization of ostrich embryos for in-ovo imaging
Abstract
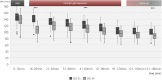
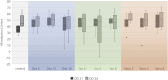
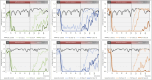
In-ovo imaging using avian eggs has been described as a potential alternative to animal testing using rodents. However, imaging studies are hampered by embryonal motion producing artifacts. This study aims at systematically comparing isoflurane, desflurane and sevoflurane in three different concentrations in ostrich embryos. Biomagnetic signals of ostrich embryos were recorded analyzing cardiac action and motion. Ten groups comprising eight ostrich embryos each were investigated: Control, isoflurane (2%, 4%, and 6%), desflurane (6%, 12%, and 18%) and sevoflurane (3%, 5%, and 8%). Each ostrich egg was exposed to the same narcotic gas and concentration on development day (DD) 31 and 34. Narcotic gas exposure was upheld for 90 min and embryos were monitored for additional 75 min. Toxicity was evaluated by verifying embryo viability 24 h after the experiments. Initial heart rate of mean 148 beats/min (DD 31) and 136 beats/min (DD 34) decreased over time by 44-48 beats/minute. No significant differences were observed between groups. All narcotic gases led to distinct movement reduction after mean 8 min. Embryos exposed to desflurane 6% showed residual movements. Isoflurane 6% and sevoflurane 8% produced motion-free time intervals of mean 70 min after discontinuation of narcotic gas exposure. Only one embryo death occurred after narcotic gas exposure with desflurane 6%. This study shows that isoflurane, desflurane and sevoflurane are suitable for ostrich embryo immobilization, which is a prerequisite for motion-artifact free imaging. Application of isoflurane 6% and sevoflurane 8% is a) safe as no embryonal deaths occurred after exposure and b) effective as immobilization was observed for approx. 70 min after the end of narcotic gas exposure. These results should be interpreted with caution regarding transferability to other avian species as differences in embryo size and incubation duration exist.
Keywords: alternative animal testing; animal model; biomagnetism; in-ovo imaging; magnet-ovography; narcotic gases; ostrich eggs.
Copyright © 2024 Perkas, Schmidt, Kuehnel, Greiser, Hermeyer, Klingner, Freesmeyer and Winkens.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
In-ovo imaging using ostrich eggs: Biomagnetism for detection of cardiac signals and embryonal motion.Exp Biol Med (Maywood). 2022 Jun;247(12):996-1004. doi: 10.1177/15353702221082046. Epub 2022 Apr 25. Exp Biol Med (Maywood). 2022. PMID: 35466741 Free PMC article.
-
Global warming potential of inhaled anesthetics: application to clinical use.Anesth Analg. 2010 Jul;111(1):92-8. doi: 10.1213/ANE.0b013e3181e058d7. Epub 2010 Jun 2. Anesth Analg. 2010. PMID: 20519425
-
Recovery and pharmacokinetic parameters of desflurane, sevoflurane, and isoflurane in patients undergoing urologic procedures.J Clin Anesth. 1999 Sep;11(6):460-5. doi: 10.1016/s0952-8180(99)00082-3. J Clin Anesth. 1999. PMID: 10526823 Clinical Trial.
-
Cardiovascular responses to sevoflurane: a review.Anesth Analg. 1995 Dec;81(6 Suppl):S11-22. doi: 10.1097/00000539-199512001-00003. Anesth Analg. 1995. PMID: 7486143 Review.
-
[Newer inhalation anaesthetics and neuro-anaesthesia: what is the place for sevoflurane or desflurane?].Ann Fr Anesth Reanim. 2004 Apr;23(4):367-74. doi: 10.1016/j.annfar.2004.01.012. Ann Fr Anesth Reanim. 2004. PMID: 15120783 Review. French.
Cited by
-
In-ovo imaging using ostrich eggs: biodistribution of F-18-FDG in ostrich embryos.Exp Biol Med (Maywood). 2025 Jun 19;250:10560. doi: 10.3389/ebm.2025.10560. eCollection 2025. Exp Biol Med (Maywood). 2025. PMID: 40612332 Free PMC article.
References
-
- Winkens T, Christl A, Kuehnel C, Ndum F, Seifert P, Greiser J, et al. In-ovo imaging using ostrich eggs-Evaluation of physiological embryonal development on computed tomography. Acta Zoologica (2021) 103:492–502. 10.1111/azo.12400 - DOI
-
- Winkens T, Kuehnel C, Freesmeyer M. In-ovo imaging using ostrich eggs - replacement of animal studies? J Nucl Med (2020) 61:1122.
-
- Leary S, Anthony R, Cartner S, Grandin T, Greenacre C, Gwaltney-Brant S, et al. AVMA guidelines for the euthanasia of animals: 2020 edition. Schaumburg, IL, USA: American Veterinary Medical Association; (2020).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

