Changes in Posttreatment Spleen Volume Associated with Immunotherapy Outcomes for Advanced Hepatocellular Carcinoma
- PMID: 38854818
- PMCID: PMC11162638
- DOI: 10.2147/JHC.S462470
Changes in Posttreatment Spleen Volume Associated with Immunotherapy Outcomes for Advanced Hepatocellular Carcinoma
Abstract
Purpose: We investigated whether spleen volume (SV) changes were associated with treatment outcomes in advanced hepatocellular carcinoma (HCC) patients who received immunotherapy or first-line sorafenib.
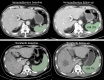
Patients and methods: Patients with advanced HCC who underwent immunotherapy or first-line sorafenib at our institute were retrospectively analyzed. CT was used to measure SV before and within 3 months of treatment initiation. Tumor assessment followed Response Evaluation Criteria in Solid Tumors version 1.1. The association between SV change and tumor response or progression-free survival (PFS) was analyzed. The inverse probability of treatment weighting (IPTW) was used to adjust for differences in baseline characteristics.
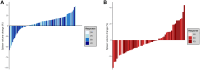
Results: The immunotherapy group comprised 143 patients (124 men, mean age, 59.8 years ± 11.2 [standard deviation]), while the sorafenib group had 57 (47 men, mean age, 59.6 years ± 9.9). SV increased in 108 (75.5%) immunotherapy and 21 (36.8%) sorafenib patients. In the immunotherapy group, patients with increased SV were more likely than those with decreased SV to have a higher disease control rate (76.9% vs 57.1%, p = 0.024) and durable clinical benefit (52.8% vs 25.7%, p = 0.005). It was also associated with extended PFS in the immunotherapy group in both the univariate (p = 0.028) and multivariate (p = 0.014) analysis. By contrast, in the sorafenib group, an increased in SV was not associated with treatment response but was presumably associated with reduced PFS (p = 0.072) in the multivariate analysis. After IPTW adjustment, the increase in SV remained a significant predictor for DCB and PFS in the immunotherapy group.
Conclusion: Most patients exhibited an increase in SV after the initiation of immunotherapy, which may be used to predict response and prognosis. However, this association was not observed in patients who received sorafenib.
Keywords: hepatocellular carcinoma; immunotherapy; response; sorafenib; survival.
Plain language summary
The study provides significant evidence that an increase in spleen volume is associated with better treatment outcomes in advanced hepatocellular carcinoma patients undergoing immunotherapy. These findings offer oncologists a new potential biomarker for optimizing treatment strategies. Specifically, increased spleen volume could be used to predict higher rates of disease control and durable clinical benefits, allowing for more personalized care.
© 2024 Chen et al.
Conflict of interest statement
Dr Chih-Hung Hsu reports grants from Roche, grants from AstraZeneca, grants from Eli Lilly, grants from Surface Oncology, personal fees from MSD, personal fees from Eisai, outside the submitted work. The authors report no other competing interests in this work.
Figures



Similar articles
-
Comparative Efficacy of Cabozantinib and Regorafenib for Advanced Hepatocellular Carcinoma.Adv Ther. 2020 Jun;37(6):2678-2695. doi: 10.1007/s12325-020-01378-y. Epub 2020 May 18. Adv Ther. 2020. PMID: 32424805 Free PMC article.
-
Baseline Splenic Volume Outweighs Immuno-Modulated Size Changes with Regard to Survival Outcome in Patients with Hepatocellular Carcinoma under Immunotherapy.Cancers (Basel). 2022 Jul 22;14(15):3574. doi: 10.3390/cancers14153574. Cancers (Basel). 2022. PMID: 35892833 Free PMC article.
-
Changes in the neutrophil-to-lymphocyte ratio predict the prognosis of patients with advanced hepatocellular carcinoma treated with sorafenib.Eur J Gastroenterol Hepatol. 2019 Oct;31(10):1250-1255. doi: 10.1097/MEG.0000000000001405. Eur J Gastroenterol Hepatol. 2019. PMID: 30925530
-
Early predictive value of circulating biomarkers for sorafenib in advanced hepatocellular carcinoma.Expert Rev Mol Diagn. 2022 Mar;22(3):361-378. doi: 10.1080/14737159.2022.2049248. Epub 2022 Mar 11. Expert Rev Mol Diagn. 2022. PMID: 35234564 Review.
-
Systemic targeted and immunotherapy for advanced hepatocellular carcinoma.Am J Health Syst Pharm. 2021 Jan 22;78(3):187-202. doi: 10.1093/ajhp/zxaa365. Am J Health Syst Pharm. 2021. PMID: 33211092 Review.
Cited by
-
Spleen Volume Dynamics and Survival Outcomes in HCC Patients Undergoing Immune Checkpoint Inhibitors: A Retrospective Analysis.J Hepatocell Carcinoma. 2025 May 30;12:1069-1082. doi: 10.2147/JHC.S524483. eCollection 2025. J Hepatocell Carcinoma. 2025. PMID: 40462878 Free PMC article.
-
The load of hepatitis B virus reduces the immune checkpoint inhibitors efficiency in hepatocellular carcinoma patients.Front Immunol. 2024 Nov 27;15:1480520. doi: 10.3389/fimmu.2024.1480520. eCollection 2024. Front Immunol. 2024. PMID: 39664382 Free PMC article.
References
-
- Shao YY, Wang SY, Lin SM, Diagnosis G, Systemic Therapy G. Management consensus guideline for hepatocellular carcinoma: 2020 update on surveillance, diagnosis, and systemic treatment by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J Formos Med Assoc. 2021;120(4):1051–1060. doi:10.1016/j.jfma.2020.10.031 - DOI - PubMed
LinkOut - more resources
Full Text Sources

