Antioxidant activity of an inclusion complex between rutin and β-cyclodextrin: experimental and quantum chemical studies
- PMID: 38854829
- PMCID: PMC11157499
- DOI: 10.1039/d4ra02307b
Antioxidant activity of an inclusion complex between rutin and β-cyclodextrin: experimental and quantum chemical studies
Abstract
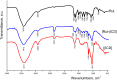
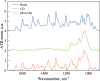
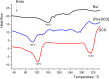
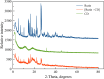
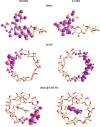
This study aims to synthesize a guest-host complex derived from rutin (Rut) and β-cyclodextrin (β-CD) (denoted as [Rut⊂β-CD]). The obtained substance was characterized by the FT-IR and DSC methods, signifying the formation of an inclusion complex between Rut and β-CD. Complex formation increased the antioxidant activity of rutin corresponding to the decrease of EC50 values from 1.547 × 10-5 mol L-1 to 1.227 × 10-5 mol L-1 according to the DPPH free radical scavenging test. The rutin-β-CD interaction energies were calculated in the vacuum and various solvents (e.g., water, ethanol, and dimethylsulfoxide) utilizing an accurate and broadly parametrized self-consistent tight-binding quantum chemical method (GFN2-xTB). The calculation results reveal the influence of solvent on the structural formation of the rutin-β-CD complex. In both the vacuum and aqueous solution, rutin can enter into the small-sized empty cavity of β-CD, albeit through different terminals, resulting in distinct preferential structures. The presence of organic solvents appears to reduce the interaction between rutin and β-CD, with the interaction strength following the order: water > ethanol > dimethyl sulfoxide.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Sofic E. Copra-Janicijevic A. Salihovic M. Tahirovic I. Kroyer G. Screening of medicinal plant extracts for quercetin-3rutinoside (rutin) in Bosnia and Herzegovina. Med. Plants – Int. J. Phytomed. Relat. 2010;2(2):97–102. doi: 10.5958/j.0975-4261.2.2.015. - DOI
-
- Le N. T. Nguyen T. P. D. Ho D. V. Phung H. T. Nguyen H. T. Green solvents-based rutin extraction from Sophora japonica L. J. Appl. Res. Med. Aromat. Plants. 2023;36:100508. doi: 10.1016/j.jarmap.2023.100508. - DOI
-
- Taraba A. Szymczyk K. Spectroscopic studies of the quercetin/rutin-nonionic surfactant interactions. J. Mol. Liq. 2022;360:119483. doi: 10.1016/j.molliq.2022.119483. - DOI
LinkOut - more resources
Full Text Sources

