Formulation of quinine suppository for initiation of early treatment of malaria - a preliminary study
- PMID: 38854879
- PMCID: PMC11153357
- DOI: 10.5281/zenodo.10997879
Formulation of quinine suppository for initiation of early treatment of malaria - a preliminary study
Abstract
Background: In the management of malaria, there is the need for early initiation of treatment. An antimalarial drug for home use must be easy to administer, safe, effective and affordable. Parenteral quinine is the gold standard for treatment of severe malaria. A rectal formulation of quinine will therefore serve the purpose of early initiation of care in patients that lack easy access to medical centers. The main objective of this preliminary work was to develop a quinine suppository with adequate release properties that also meets the dual conditions of affordability and ease of administration.
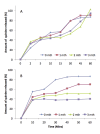
Materials and methods: Cocoa butter and Fattibase™ were used in the preparation of suppositories containing 200 mg quinine bisulphate. The release profiles of formulations with varying concentrations of polysorbate 80 (0 - 5%) were evaluated by in vitro dissolution in pH 8 buffer medium.
Results: The addition of polysorbate 80 improved the release of quinine significantly at 2 and 5%. Cocoa butter suppository with 1% polysorbate 80 released 73.6 mg quinine bisulphate in 1 hr while release from suppositories with 2% and 5% surfactant was higher. Fattibase™ suppositories had better release profiles than cocoa butter formulations. The formulation with 5% polysorbate 80 released 170 mg quinine in 1 hr. Formulations with the two bases released quinine in adequate quantities for the management of malaria.
Conclusions: The particle size of quinine is an important factor affecting the physical appearance and drug release from the suppository. The Fattibase™ suppositories were more stable but cost five times the price of the cocoa butter formulations. The cocoa butter formulations, however, still released quinine in sufficient quantities for the management of malaria. Cocoa butter formulations will be more affordable in resource-limited malaria-endemic regions of the world.
Copyright © 2012: Soremekun et al.
Conflict of interest statement
Competing interests: No competing interests declared.
Figures

Similar articles
-
Isoniazid release from suppositories compounded with selected bases.Int J Pharm Compd. 2007 Sep-Oct;11(5):433-7. Int J Pharm Compd. 2007. PMID: 23969524
-
Ketorolac tromethamine and ketoprofen suppositories: release profiles and bioavailability of a cocoa butter base formula in rabbits.Int J Pharm Compd. 1998 Sep-Oct;2(5):390-3. Int J Pharm Compd. 1998. PMID: 23989705
-
Formulation and Evaluation of Tramadol hydrochloride Rectal Suppositories.Indian J Pharm Sci. 2008 Sep;70(5):640-4. doi: 10.4103/0250-474X.45405. Indian J Pharm Sci. 2008. PMID: 21394263 Free PMC article.
-
Designing and developing suppository formulations for anti-HIV drug delivery.Ther Deliv. 2017 Aug;8(9):805-817. doi: 10.4155/tde-2017-0056. Ther Deliv. 2017. PMID: 28825395 Free PMC article. Review.
-
Artesunate suppositories: an effective treatment for severe falciparum malaria in rural areas.Ann Trop Med Parasitol. 1997 Oct;91(7):891-6. doi: 10.1080/00034989760662. Ann Trop Med Parasitol. 1997. PMID: 9625947 Review.
References
-
- Muranishi S. Characteristics of drug absorption via the rectal route. Methods Find. Exp. Clin. Pharmacol. 1984;6:763–772. - PubMed
-
- Taylor O, Igwilo CI, Silva BO, Soremekun R, Dada AA. Stability studies of Paracetamol suppositories formulated with polyethylene glycols. J. West Afr. Pharm. 1993;7:19.
-
- De Boer AG, Moolenaar F, DeLeede LG, Breimer DD. Rectal drug administration: clinical pharmacokinetic considerations. Clin. Pharmacokinet. 1982;7:285–311. - PubMed
-
- Berko S, Regdon G Jr, Ducza E, Falkay G, Eros I. In vitro and in vivo study in rats of rectal suppositories containing furosemide. Eur. J. Pharm. Biopharm. 2002;53:311–315. - PubMed
-
- Realdon N, Ragazzi EU, Ragazzi EN. Effect of drug solubility on in vitro availability rate from suppositories with lipophilic excipients. Pharmazie. 2000;55:372–377. - PubMed
LinkOut - more resources
Full Text Sources
