Review of Opioid Abuse-Deterrent Formulations: Impact and Barriers to Access
- PMID: 38854928
- PMCID: PMC11162618
- DOI: 10.2147/JPR.S457982
Review of Opioid Abuse-Deterrent Formulations: Impact and Barriers to Access
Abstract
The misuse and abuse of opioid analgesics continue to pose a serious public health concern, but for some patients, opioids remain an important analgesic option. Extended-release (ER) opioid formulations are effective for treating chronic pain and are supported by multiple 12-week efficacy studies. ER opioids often contain a high opioid content, and similar to immediate-release (IR) formulations, are subject to abuse, misuse, and diversion. Unintentional misuse may also occur when ER formulations are manipulated for medicinal administration, such as crushing a dose for easier oral intake. As part of a multipronged strategy designed to fight the opioid epidemic, abuse-deterrent formulations (ADFs) were developed to deter misuse, abuse, and diversion of opioids by making manipulation more difficult and nonoral routes of administration less rewarding. Although ADF opioids have been shown to decrease rates of abuse and diversion, they are not equally effective in terms of deterring manipulation for abuse or misuse. Xtampza ER utilizes DETERx technology, which allows it to retain ER characteristics when chewed or crushed, making it the only ER opioid without a boxed warning against these types of manipulation. OxyContin was also developed as an ADF but uses RESISTEC technology, making the tablet hard to crush and viscous in aqueous solutions. ADF utilization has been hampered by patient access issues, including high prices due to lack of insurance coverage. Postmarket real-world studies demonstrate lower rates of abuse, misuse, and diversion for ADF ER opioids compared with non-ADF formulations. However, similar studies comparing abuse-related effectiveness and health care costs for ADF opioids are warranted if clinicians are expected to utilize these potentially safer opioid formulations. These studies would support further education surrounding the benefits and utilization of ADFs and manipulation potential of different ADFs.
Keywords: abuse-deterrent; chronic pain; extended release; opioid analgesics; opioid crisis; tamper.
© 2024 Webster and Gudin.
Conflict of interest statement
LW is a consultant for CognifiSense, Collegium, Elysium Pharmaceuticals, Ensysce Biosciences, Quivive Pharma, Salix Pharmaceuticals, Trevi Therapeutics; advisory board of AdhereRx, Ensysce Biosciences, KemPharm, MedLogix; travel expenses from AdhereRx, Elysium Pharmaceuticals, Ensysce Biosciences, PainScript. JG is a consultant for Collegium, Hisamitsu, Kailo, Protega, Quest Diagnostics, Sanofi; shareholder for Virpax. The authors report no other conflicts of interest in this work.
Figures

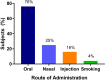
References
-
- US Food and Drug Administration. Center for drug evaluation and research. General principles for evaluating the abuse deterrence of generic solid oral opioid drug products guidance for industry. Available from: https://www.fda.gov/media/96643/download. Accessed July 24, 2023.
-
- Data Analysis & Resources. US center for disease control and prevention; 2022. Available from: https://www.cdc.gov/opioids/data/analysis-resources.html. Accessed July 24, 2023.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

