A rare case report of a primary lung cancer comprising adenocarcinoma and atypical carcinoid tumor, with the carcinoid component harboring EML4-ALK rearrangement
- PMID: 38854939
- PMCID: PMC11157374
- DOI: 10.21037/tlcr-24-352
A rare case report of a primary lung cancer comprising adenocarcinoma and atypical carcinoid tumor, with the carcinoid component harboring EML4-ALK rearrangement
Abstract
Background: The occurrence of pulmonary adenocarcinoma coexisting with atypical carcinoid tumors is a rare phenomenon. The presence of EML4-ALK fusion in an atypical carcinoid component of a histologically mixed tumor is even more uncommon. Due to their infrequency, the origin and pathogenesis of these mixed tumors remain largely unknown. The advances of therapy development in such patients are still limited and there is no standard treatment. We present a case of collision tumor in the lung consisting of atypical carcinoid and adenocarcinoma to better understand the clinical characteristics of this disease.
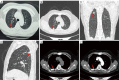
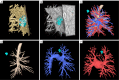
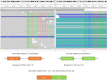
Case description: We report an extremely rare case of EML4-ALK rearrangement in a pulmonary atypical carcinoid tumor that coexisting with adenocarcinoma. A 58-year-old woman, who was asymptomatic, underwent pulmonary lobectomy due to the detection of a gradually enlarging solitary pulmonary nodule in the right upper lung. Histological examination of the resected tumor revealed the presence of both atypical carcinoid (approximately 80%) and adenocarcinoma (approximately 20%) components. Metastases by the carcinoid component were observed in mediastinal lymph nodes (station 2R and 4R) and in the primary tumor. Anaplastic lymphoma kinase (ALK) rearrangement was detected in both the primary and metastatic lesions of the carcinoid tumor. Four cycles of chemotherapy with etoposide and carboplatin were dispensed after surgery.
Conclusions: This is the first reported case of coexisting pulmonary adenocarcinoma and atypical carcinoid tumor with an ALK fusion only detected in the carcinoid component. The presence of ALK rearrangement in pulmonary carcinoid tumor is very uncommon, and there is currently no standard treatment for advanced stages. Therefore, comprehensive molecular testing, including ALK rearrangement analysis, should be recommended for mixed tumors exhibiting features of atypical carcinoid. ALK inhibitors could represent a potential treatment strategy for selected patients.
Keywords: ALK rearrangement; case report; pulmonary adenocarcinoma; pulmonary atypical carcinoid.
2024 Translational Lung Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-352/coif). R.A.R. and T.H. serve as unpaid editorial board members of Translational Lung Cancer Research from January 2024 to December 2025. J.S. is from Dagong Law Firm. E.M.U. received grants from Merck and AstraZeneca; honoraria from Janssen, Amgen, AstraZeneca, Novartis; support for attending meetings and travel from AstraZeneca and Roche; payment for participation in Advisory Board from Roche, Takeda, Pfizer, AstraZeneca. E.S.R. received grants from Sanofi and Takeda; honoraria from Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Roche, Takeda; payment for participation in Advisory Board from Roche and Takeda. P.C. has received research funding from AstraZeneca, Amgen, Boehringer Ingelheim, Merck, Novartis, Roche, and Takeda, speaker’s honoraria from AstraZeneca, Gilead, Janssen, Novartis, Roche, Pfizer, Thermo Fisher, Takeda, support for attending meetings from AstraZeneca, Eli Lilly, Daiichi Sankyo, Janssen, Gilead, Novartis, Pfizer, Takeda, and personal fees for participating to advisory boards from AstraZeneca, Boehringer Ingelheim, Chugai, Pfizer, Novartis, MSD, Takeda and Roche, all outside the submitted work. R.A.R. has consulting agreements with several companies (TerSera Therapeutics, ITM Radiopharma, Regeneron, Advanced Accelerator Applications, Novartis, Ipsen, Amgen, Astra-Zeneca, Curium, Exelexis, EMD Serono) that manufacture products to treat neuroendocrine tumors and lung cancers, all outside the submitted work. R.A.R. is a Board of Directors member of the North American Neuroendocrine Tumor Society. The other authors have no conflicts of interest to declare.
Figures



Similar articles
-
A case of primary pulmonary atypical carcinoid with EML4-ALK rearrangement.Cancer Biol Ther. 2020;21(1):12-16. doi: 10.1080/15384047.2019.1665957. Epub 2019 Sep 27. Cancer Biol Ther. 2020. PMID: 31559892 Free PMC article.
-
Metastatic pulmonary carcinoids with EML4-ALK fusion response to ALK inhibitors: two case reports and review of literature.Transl Lung Cancer Res. 2022 Jun;11(6):1176-1184. doi: 10.21037/tlcr-22-394. Transl Lung Cancer Res. 2022. PMID: 35832448 Free PMC article.
-
Atypical Lung Carcinoid With EML4/ALK Fusion Detected With Circulating Tumor DNA.Cureus. 2022 Feb 16;14(2):e22276. doi: 10.7759/cureus.22276. eCollection 2022 Feb. Cureus. 2022. PMID: 35350512 Free PMC article.
-
Transformation of NSCLC to SCLC harboring EML4-ALK fusion with V1180L mutation after alectinib resistance and response to lorlatinib: A case report and literature review.Lung Cancer. 2023 Dec;186:107415. doi: 10.1016/j.lungcan.2023.107415. Epub 2023 Oct 28. Lung Cancer. 2023. PMID: 37907052 Review.
-
ALK inhibitors in the treatment of advanced NSCLC.Cancer Treat Rev. 2014 Mar;40(2):300-6. doi: 10.1016/j.ctrv.2013.07.002. Epub 2013 Aug 7. Cancer Treat Rev. 2014. PMID: 23931927 Review.
Cited by
-
Basic science and translational implications of current knowledge on neuroendocrine tumors.J Clin Invest. 2025 Mar 3;135(5):e186702. doi: 10.1172/JCI186702. J Clin Invest. 2025. PMID: 40026252 Free PMC article. Review.
References
-
- WHO Classification of Tumours Editorial Board. WHO classification of tumours. Thoracic tumours.5th ed. Lyon: IARC Press; 2021.
Publication types
LinkOut - more resources
Full Text Sources
