Gene expression and ultra-structural evidence for metabolic derangement in the primary mitral regurgitation heart
- PMID: 38854954
- PMCID: PMC11157345
- DOI: 10.1093/ehjopen/oeae034
Gene expression and ultra-structural evidence for metabolic derangement in the primary mitral regurgitation heart
Abstract
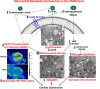
Aims: Chronic neurohormonal activation and haemodynamic load cause derangement in the utilization of the myocardial substrate. In this study, we test the hypothesis that the primary mitral regurgitation (PMR) heart shows an altered metabolic gene profile and cardiac ultra-structure consistent with decreased fatty acid and glucose metabolism despite a left ventricular ejection fraction (LVEF) > 60%.
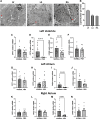
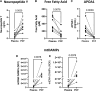
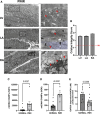
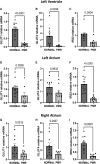
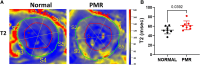
Methods and results: Metabolic gene expression in right atrial (RA), left atrial (LA), and left ventricular (LV) biopsies from donor hearts (n = 10) and from patients with moderate-to-severe PMR (n = 11) at surgery showed decreased mRNA glucose transporter type 4 (GLUT4), GLUT1, and insulin receptor substrate 2 and increased mRNA hexokinase 2, O-linked N-acetylglucosamine transferase, and O-linked N-acetylglucosaminyl transferase, rate-limiting steps in the hexosamine biosynthetic pathway. Pericardial fluid levels of neuropeptide Y were four-fold higher than simultaneous plasma, indicative of increased sympathetic drive. Quantitative transmission electron microscopy showed glycogen accumulation, glycophagy, increased lipid droplets (LDs), and mitochondrial cristae lysis. These findings are associated with increased mRNA for glycogen synthase kinase 3β, decreased carnitine palmitoyl transferase 2, and fatty acid synthase in PMR vs. normals. Cardiac magnetic resonance and positron emission tomography for 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG) uptake showed decreased LV [18F]FDG uptake and increased plasma haemoglobin A1C, free fatty acids, and mitochondrial damage-associated molecular patterns in a separate cohort of patients with stable moderate PMR with an LVEF > 60% (n = 8) vs. normal controls (n = 8).
Conclusion: The PMR heart has a global ultra-structural and metabolic gene expression pattern of decreased glucose uptake along with increased glycogen and LDs. Further studies must determine whether this presentation is an adaptation or maladaptation in the PMR heart in the clinical evaluation of PMR.
Keywords: Lipid and glucose metabolism; Mitochondria dysfunction; Primary mitral regurgitation; [18F]FDG-PET/CMR.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: Authors declare no conflict of interest.
Figures


Similar articles
-
Positron emission tomography for the assessment of myocardial viability: an evidence-based analysis.Ont Health Technol Assess Ser. 2005;5(16):1-167. Epub 2005 Oct 1. Ont Health Technol Assess Ser. 2005. PMID: 23074467 Free PMC article.
-
Elevated cardiac hemoglobin expression is associated with a pro-oxidative and inflammatory environment in primary mitral regurgitation.Free Radic Biol Med. 2023 Nov 1;208:126-133. doi: 10.1016/j.freeradbiomed.2023.08.007. Epub 2023 Aug 3. Free Radic Biol Med. 2023. PMID: 37543167
-
ACRIN 6665/RTOG 0132 phase II trial of neoadjuvant imatinib mesylate for operable malignant gastrointestinal stromal tumor: monitoring with 18F-FDG PET and correlation with genotype and GLUT4 expression.J Nucl Med. 2012 Apr;53(4):567-74. doi: 10.2967/jnumed.111.094425. Epub 2012 Mar 1. J Nucl Med. 2012. PMID: 22381410 Free PMC article. Clinical Trial.
-
Understanding post-surgical decline in left ventricular function in primary mitral regurgitation using regression and machine learning models.Front Cardiovasc Med. 2023 Apr 21;10:1112797. doi: 10.3389/fcvm.2023.1112797. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37153472 Free PMC article.
-
Imaging of cardiac metabolism using radiolabelled glucose, fatty acids and acetate.Coron Artery Dis. 2001 Feb;12 Suppl 1:S12-8. Coron Artery Dis. 2001. PMID: 11286301 Review.
Cited by
-
Involvement of Oxidative Stress in Mitochondrial Abnormalities During the Development of Heart Disease.Biomedicines. 2025 May 29;13(6):1338. doi: 10.3390/biomedicines13061338. Biomedicines. 2025. PMID: 40564057 Free PMC article. Review.
References
-
- Miller JD, Suri RM. Left ventricular dysfunction after degenerative mitral valve repair: a question of better molecular targets or better surgical timing? J Thorac Cardiovasc Surg 2016;152:1071–1074. - PubMed
-
- Ahmed MI, Gladden JD, Litovsky SH, Lloyd SG, Gupta H, Inusah S, Denney T, Powell P, McGiffin DC, Dell’Italia LJ. Increased oxidative stress and cardiomyocyte myofibrillar degeneration in patients with chronic isolated mitral regurgitation and ejection fraction >60%. J Am Coll Cardiol 2010;55:671–679. - PMC - PubMed
-
- Ahmed MI, Guichard JL, Soorappan RN, Ahmad S, Mariappan N, Litovsky S, Gupta H, Lloyd SG, Denney TS, Powell PC, Aban I, Collawn J, Davies JE, McGiffin DC, Dell’Italia LJ. Disruption of desmin-mitochondrial architecture in patients with regurgitant mitral valves and preserved ventricular function. J Thorac Cardiovasc Surg 2016;152:1059–1070.e2. - PMC - PubMed
-
- Butts B, Ahmed MI, Powell PC, Pat B, Litovsky S, Gupta H, Lloyd SG, Denney TS, Zhang X, Aban I, Sadayappan S, McNamara JW, Watson MJ, Ferrario CM, Collawn JF, Lewis C, Davies JE, Dell’Italia LJ.. Left atrial emptying fraction and chymase activation in the pathophysiology of primary mitral regurgitation. JACC Basic Transl Sci 2020;5:109–122. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

