Simultaneous targeting of Tim3 and A2a receptors modulates MSLN-CAR T cell antitumor function in a human cervical tumor xenograft model
- PMID: 38855110
- PMCID: PMC11157064
- DOI: 10.3389/fimmu.2024.1362904
Simultaneous targeting of Tim3 and A2a receptors modulates MSLN-CAR T cell antitumor function in a human cervical tumor xenograft model
Abstract
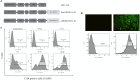
Introduction: Chimeric antigen receptor (CAR) T cell therapy has transformed the treatment of hematological malignancies. However, its efficacy in solid tumors is limited by the immunosuppressive tumor microenvironment that compromises CAR T cell antitumor function in clinical settings. To overcome this challenge, researchers have investigated the potential of inhibiting specific immune checkpoint receptors, including A2aR (Adenosine A2 Receptor) and Tim3 (T cell immunoglobulin and mucin domain-containing protein 3), to enhance CAR T cell function. In this study, we evaluated the impact of genetic targeting of Tim3 and A2a receptors on the antitumor function of human mesothelin-specific CAR T cells (MSLN-CAR) in vitro and in vivo.
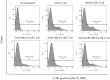
Methods: Second-generation anti-mesothelin CAR T cells were produced using standard cellular and molecular techniques. A2aR-knockdown and/or Tim3- knockdown anti-mesothelin-CAR T cells were generated using shRNA-mediated gene silencing. The antitumor function of CAR T cells was evaluated by measuring cytokine production, proliferation, and cytotoxicity in vitro through coculture with cervical cancer cells (HeLa cell line). To evaluate in vivo antitumor efficacy of manufactured CAR T cells, tumor growth and mouse survival were monitored in a human cervical cancer xenograft model.
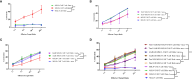
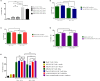
Results: In vitro experiments demonstrated that knockdown of A2aR alone or in combination with Tim3 significantly improved CAR T cell proliferation, cytokine production, and cytotoxicity in presence of tumor cells in an antigen-specific manner. Furthermore, in the humanized xenograft model, both double knockdown CAR T cells and control CAR T cells could effectively control tumor growth. However, single knockdown CAR T cells were associated with reduced survival in mice.
Conclusion: These findings highlight the potential of concomitant genetic targeting of Tim3 and A2a receptors to augment the efficacy of CAR T cell therapy in solid tumors. Nevertheless, caution should be exercised in light of our observation of decreased survival in mice treated with single knockdown MSLN-CAR T cells, emphasizing the need for careful efficacy considerations.
Keywords: A2aR; CAR T cell therapy; Tim3; genetic targeting; shRNA; solid tumors; xenograft.
Copyright © 2024 Soltantoyeh, Akbari, Shahosseini, Mirzaei and Hadjati.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Disruption of adenosine 2A receptor improves the anti-tumor function of anti-mesothelin CAR T cells both in vitro and in vivo.Exp Cell Res. 2021 Dec 1;409(1):112886. doi: 10.1016/j.yexcr.2021.112886. Epub 2021 Oct 19. Exp Cell Res. 2021. PMID: 34673000
-
Targeted knockdown of Tim3 by short hairpin RNAs improves the function of anti-mesothelin CAR T cells.Mol Immunol. 2021 Nov;139:1-9. doi: 10.1016/j.molimm.2021.06.007. Epub 2021 Aug 25. Mol Immunol. 2021. PMID: 34450537
-
Genetic and pharmacological targeting of A2a receptor improves function of anti-mesothelin CAR T cells.J Exp Clin Cancer Res. 2020 Mar 10;39(1):49. doi: 10.1186/s13046-020-01546-6. J Exp Clin Cancer Res. 2020. PMID: 32151275 Free PMC article.
-
CAR T cells in lung cancer: Targeting tumor-associated antigens to revolutionize immunotherapy.Pathol Res Pract. 2025 May;269:155947. doi: 10.1016/j.prp.2025.155947. Epub 2025 Mar 29. Pathol Res Pract. 2025. PMID: 40168775 Review.
-
Revolutionizing pancreatic cancer treatment with CAR-T therapy.Adv Protein Chem Struct Biol. 2025;144:331-353. doi: 10.1016/bs.apcsb.2024.10.008. Epub 2025 Jan 31. Adv Protein Chem Struct Biol. 2025. PMID: 39978971 Review.
Cited by
-
Neoantigen mRNA vaccines and A2A receptor antagonism: A strategy to enhance T cell immunity.Hum Vaccin Immunother. 2025 Dec;21(1):2458936. doi: 10.1080/21645515.2025.2458936. Epub 2025 Jan 30. Hum Vaccin Immunother. 2025. PMID: 39882781 Free PMC article. Review.
-
Precision oncolytic viral therapy in colorectal cancer: Genetic targeting and immune modulation for personalized treatment (Review).Int J Mol Med. 2025 Jul;56(1):104. doi: 10.3892/ijmm.2025.5545. Epub 2025 May 9. Int J Mol Med. 2025. PMID: 40342021 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

