Oral curcumin ameliorates acute murine campylobacteriosis
- PMID: 38855111
- PMCID: PMC11157060
- DOI: 10.3389/fimmu.2024.1363457
Oral curcumin ameliorates acute murine campylobacteriosis
Abstract
Introduction: Human infections with the food-borne enteropathogen Campylobacter jejuni are responsible for increasing incidences of acute campylobacteriosis cases worldwide. Since antibiotic treatment is usually not indicated and the severity of the enteritis directly correlates with the risk of developing serious autoimmune disease later-on, novel antibiotics-independent intervention strategies with non-toxic compounds to ameliorate and even prevent campylobacteriosis are utmost wanted. Given its known pleiotropic health-promoting properties, curcumin constitutes such a promising candidate molecule. In our actual preclinical placebo-controlled intervention trial, we tested the anti-microbial and anti-inflammatory effects of oral curcumin pretreatment during acute experimental campylobacteriosis.
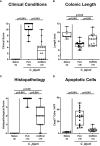
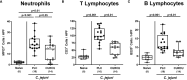
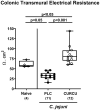
Methods: Therefore, secondary abiotic IL-10-/- mice were challenged with synthetic curcumin via the drinking water starting a week prior oral C. jejuni infection. To assess anti-pathogenic, clinical, immune-modulatory, and functional effects of curcumin prophylaxis, gastrointestinal C. jejuni bacteria were cultured, clinical signs and colonic histopathological changes quantitated, pro-inflammatory immune cell responses determined by in situ immunohistochemistry and intestinal, extra-intestinal and systemic pro-inflammatory mediator measurements, and finally, intestinal epithelial barrier function tested by electrophysiological resistance analysis of colonic ex vivo biopsies in the Ussing chamber.
Results and discussion: Whereas placebo counterparts were suffering from severe enterocolitis characterized by wasting symptoms and bloody diarrhea on day 6 post-infection, curcumin pretreated mice, however, were clinically far less compromised and displayed less severe microscopic inflammatory sequelae such as histopathological changes and epithelial cell apoptosis in the colon. In addition, curcumin pretreatment could mitigate pro-inflammatory innate and adaptive immune responses in the intestinal tract and importantly, rescue colonic epithelial barrier integrity upon C. jejuni infection. Remarkably, the disease-mitigating effects of exogenous curcumin was also observed in organs beyond the infected intestines and strikingly, even systemically given basal hepatic, renal, and serum concentrations of pro-inflammatory mediators measured in curcumin pretreated mice on day 6 post-infection. In conclusion, the anti-Campylobacter and disease-mitigating including anti-inflammatory effects upon oral curcumin application observed here highlight the polyphenolic compound as a promising antibiotics-independent option for the prevention from severe acute campylobacteriosis and its potential post-infectious complications.
Keywords: Campylobacter jejuni; acute enterocolitis; campylobacteriosis model; curcumin; host-pathogen interaction; polyphenols; preclinical placebo-controlled intervention study; secondary abiotic IL-10 -/-mice.
Copyright © 2024 Heimesaat, Mousavi, Lobo de Sá, Peh, Schulzke, Bücker, Kittler and Bereswill.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

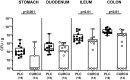


Similar articles
-
Vitamin D in Acute Campylobacteriosis-Results From an Intervention Study Applying a Clinical Campylobacter jejuni Induced Enterocolitis Model.Front Immunol. 2019 Sep 3;10:2094. doi: 10.3389/fimmu.2019.02094. eCollection 2019. Front Immunol. 2019. PMID: 31552040 Free PMC article.
-
Menthol Pretreatment Alleviates Campylobacter jejuni-Induced Enterocolitis in Human Gut Microbiota-Associated IL-10-/- Mice.Biomolecules. 2024 Feb 29;14(3):290. doi: 10.3390/biom14030290. Biomolecules. 2024. PMID: 38540710 Free PMC article.
-
Prophylactic Oral Application of Activated Charcoal Mitigates Acute Campylobacteriosis in Human Gut Microbiota-Associated IL-10-/- Mice.Biomolecules. 2024 Jan 23;14(2):141. doi: 10.3390/biom14020141. Biomolecules. 2024. PMID: 38397378 Free PMC article.
-
Novel Clinical Campylobacter jejuni Infection Models Based on Sensitization of Mice to Lipooligosaccharide, a Major Bacterial Factor Triggering Innate Immune Responses in Human Campylobacteriosis.Microorganisms. 2020 Mar 28;8(4):482. doi: 10.3390/microorganisms8040482. Microorganisms. 2020. PMID: 32231139 Free PMC article. Review.
-
Human Campylobacteriosis-A Serious Infectious Threat in a One Health Perspective.Curr Top Microbiol Immunol. 2021;431:1-23. doi: 10.1007/978-3-030-65481-8_1. Curr Top Microbiol Immunol. 2021. PMID: 33620646 Review.
Cited by
-
Therapeutic and protective approaches to combat Campylobacter jejuni infections.Front Pharmacol. 2025 May 6;16:1572616. doi: 10.3389/fphar.2025.1572616. eCollection 2025. Front Pharmacol. 2025. PMID: 40395728 Free PMC article. Review.
References
-
- WHO. World Health Organisation . Campylobacter (2020). Available online at: https://www.who.int/news-room/fact-sheets/detail/campylobacter.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

