Palmitate and group B Streptococcus synergistically and differentially induce IL-1β from human gestational membranes
- PMID: 38855112
- PMCID: PMC11158625
- DOI: 10.3389/fimmu.2024.1409378
Palmitate and group B Streptococcus synergistically and differentially induce IL-1β from human gestational membranes
Abstract
Introduction: Rupture of the gestational membranes often precedes major pregnancy complications, including preterm labor and preterm birth. One major cause of inflammation in the gestational membranes, chorioamnionitis (CAM) is often a result of bacterial infection. The commensal bacterium Streptococcus agalactiae, or Group B Streptococcus (GBS) is a leading infectious cause of CAM. Obesity is on the rise worldwide and roughly 1 in 4 pregnancy complications is related to obesity, and individuals with obesity are also more likely to be colonized by GBS. The gestational membranes are comprised of several distinct cell layers which are, from outermost to innermost: maternally-derived decidual stromal cells (DSCs), fetal cytotrophoblasts (CTBs), fetal mesenchymal cells, and fetal amnion epithelial cells (AECs). In addition, the gestational membranes have several immune cell populations; macrophages are the most common phagocyte. Here we characterize the effects of palmitate, the most common long-chain saturated fatty acid, on the inflammatory response of each layer of the gestational membranes when infected with GBS, using human cell lines and primary human tissue.
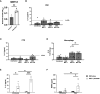
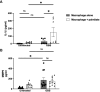
Results: Palmitate itself slightly but significantly augments GBS proliferation. Palmitate and GBS co-stimulation synergized to induce many inflammatory proteins and cytokines, particularly IL-1β and matrix metalloproteinase 9 from DSCs, CTBs, and macrophages, but not from AECs. Many of these findings are recapitulated when treating cells with palmitate and a TLR2 or TLR4 agonist, suggesting broad applicability of palmitate-pathogen synergy. Co-culture of macrophages with DSCs or CTBs, upon co-stimulation with GBS and palmitate, resulted in increased inflammatory responses, contrary to previous work in the absence of palmitate. In whole gestational membrane biopsies, the amnion layer appeared to dampen immune responses from the DSC and CTB layers (the choriodecidua) to GBS and palmitate co-stimulation. Addition of the monounsaturated fatty acid oleate, the most abundant monounsaturated fatty acid in circulation, dampened the proinflammatory effect of palmitate.
Discussion: These studies reveal a complex interplay between the immunological response of the distinct layers of the gestational membrane to GBS infection and that such responses can be altered by exposure to long-chain saturated fatty acids. These data provide insight into how metabolic syndromes such as obesity might contribute to an increased risk for GBS disease during pregnancy.
Keywords: gestational membranes; group B Streptococcus; inflammation; obesity; palmitate; pregnancy; preterm birth; preterm prelabor rupture of membranes.
Copyright © 2024 Gaddy, Moore, Lochner, Rogers, Noble, Giri, Aronoff, Cliffel and Eastman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures





Similar articles
-
Cytotrophoblasts suppress macrophage-mediated inflammation through a contact-dependent mechanism.Am J Reprod Immunol. 2021 Mar;85(3):e13352. doi: 10.1111/aji.13352. Epub 2020 Oct 16. Am J Reprod Immunol. 2021. PMID: 32969101 Free PMC article.
-
Streptococcus agalactiae Induces Placental Macrophages To Release Extracellular Traps Loaded with Tissue Remodeling Enzymes via an Oxidative Burst-Dependent Mechanism.mBio. 2018 Nov 20;9(6):e02084-18. doi: 10.1128/mBio.02084-18. mBio. 2018. PMID: 30459195 Free PMC article.
-
Decidual stromal cell-derived PGE2 regulates macrophage responses to microbial threat.Am J Reprod Immunol. 2018 Oct;80(4):e13032. doi: 10.1111/aji.13032. Epub 2018 Aug 7. Am J Reprod Immunol. 2018. PMID: 30084522 Free PMC article.
-
The prevention of early-onset neonatal group B streptococcal disease.J Obstet Gynaecol Can. 2013 Oct;35(10):939-948. doi: 10.1016/S1701-2163(15)30818-5. J Obstet Gynaecol Can. 2013. PMID: 24165063 Review.
-
Mechanisms of group B Streptococcus-mediated preterm birth: lessons learnt from animal models.Reprod Fertil. 2022 Jun 7;3(3):R109-R120. doi: 10.1530/RAF-21-0105. eCollection 2022 Jul 1. Reprod Fertil. 2022. PMID: 35794927 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

