PSMA-targeted radiotheranostics in modern nuclear medicine: then, now, and what of the future?
- PMID: 38855174
- PMCID: PMC11155394
- DOI: 10.7150/thno.92612
PSMA-targeted radiotheranostics in modern nuclear medicine: then, now, and what of the future?
Abstract
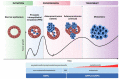
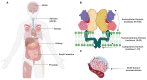
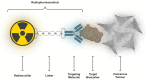
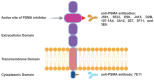
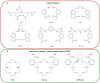
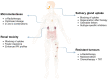
In 1853, the perception of prostate cancer (PCa) as a rare ailment prevailed, was described by the eminent Londoner surgeon John Adams. Rapidly forward to 2018, the landscape dramatically altered. Currently, men face a one-in-nine lifetime risk of PCa, accentuated by improved diagnostic methods and an ageing population. With more than three million men in the United States alone grappling with this disease, the overall risk of succumbing to stands at one in 39. The intricate clinical and biological diversity of PCa poses serious challenges in terms of imaging, ongoing monitoring, and disease management. In the field of theranostics, diagnostic and therapeutic approaches that harmoniously merge targeted imaging with treatments are integrated. A pivotal player in this arena is radiotheranostics, employing radionuclides for both imaging and therapy, with prostate-specific membrane antigen (PSMA) at the forefront. Clinical milestones have been reached, including FDA- and/or EMA-approved PSMA-targeted radiodiagnostic agents, such as [18F]DCFPyL (PYLARIFY®, Lantheus Holdings), [18F]rhPSMA-7.3 (POSLUMA®, Blue Earth Diagnostics) and [68Ga]Ga-PSMA-11 (Locametz®, Novartis/ ILLUCCIX®, Telix Pharmaceuticals), as well as PSMA-targeted radiotherapeutic agents, such as [177Lu]Lu-PSMA-617 (Pluvicto®, Novartis). Concurrently, ligand-drug and immune therapies designed to target PSMA are being advanced through rigorous preclinical research and clinical trials. This review delves into the annals of PSMA-targeted radiotheranostics, exploring its historical evolution as a signature molecule in PCa management. We scrutinise its clinical ramifications, acknowledge its limitations, and peer into the avenues that need further exploration. In the crucible of scientific inquiry, we aim to illuminate the path toward a future where the enigma of PCa is deciphered and where its menace is met with precise and effective countermeasures. In the following sections, we discuss the intriguing terrain of PCa radiotheranostics through the lens of PSMA, with the fervent hope of advancing our understanding and enhancing clinical practice.
Keywords: Antibodies; Inhibitors; Metastatic castration-resistant prostate cancer; Metastatic hormone-sensitive prostate cancer; Nanoparticles; Nuclear medicine; PSMA-targeted theranostics; Prostate cancer; Prostate-specific membrane antigen; Radiotheranostics.
© The author(s).
Conflict of interest statement
Competing Interests: Martina Benešová-Schäfer is listed as a coinventor in various patents on PSMA ligands. The other co-authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Radionuclides Landscape in Prostate Cancer Theranostics.Int J Mol Sci. 2025 Jul 14;26(14):6751. doi: 10.3390/ijms26146751. Int J Mol Sci. 2025. PMID: 40724999 Free PMC article. Review.
-
Advances in prostate-specific membrane antigen-targeted theranostics: from radionuclides to near-infrared fluorescence technology.Front Immunol. 2025 Jan 10;15:1533532. doi: 10.3389/fimmu.2024.1533532. eCollection 2024. Front Immunol. 2025. PMID: 39867892 Free PMC article. Review.
-
Evaluation of online teaching modules for PSMA PET interpretation.Prostate. 2024 Dec;84(16):1419-1426. doi: 10.1002/pros.24780. Epub 2024 Sep 9. Prostate. 2024. PMID: 39246039
-
Dansylated Amino Acid-Modified Long-Acting PSMA Derivatives 68Ga/177Lu-LNC1011 as Prostate Cancer Theranostics.J Nucl Med. 2025 May 1;66(5):739-747. doi: 10.2967/jnumed.124.268959. J Nucl Med. 2025. PMID: 40113222
-
Theranostics for Advanced Prostate Cancer: Current Indications and Future Developments.Eur Urol Oncol. 2019 Mar;2(2):152-162. doi: 10.1016/j.euo.2019.01.001. Epub 2019 Jan 31. Eur Urol Oncol. 2019. PMID: 31017091 Review.
Cited by
-
Periprostatic adipose tissue inhibits tumor progression by secreting apoptotic factors: A natural barrier induced by the immune response during the early stages of prostate cancer.Oncol Lett. 2024 Aug 8;28(4):485. doi: 10.3892/ol.2024.14617. eCollection 2024 Oct. Oncol Lett. 2024. PMID: 39170882 Free PMC article.
-
Prostate Cancer: Emerging Modifiable Risk Factors and Therapeutic Strategies in the Management of Advanced Cancer.Life (Basel). 2024 Aug 30;14(9):1094. doi: 10.3390/life14091094. Life (Basel). 2024. PMID: 39337878 Free PMC article. Review.
-
Future Prospect of Low-Molecular-Weight Prostate-Specific Membrane Antigen Radioisotopes Labeled as Theranostic Agents for Metastatic Castration-Resistant Prostate Cancer.Molecules. 2024 Dec 23;29(24):6062. doi: 10.3390/molecules29246062. Molecules. 2024. PMID: 39770150 Free PMC article. Review.
-
Radionuclides Landscape in Prostate Cancer Theranostics.Int J Mol Sci. 2025 Jul 14;26(14):6751. doi: 10.3390/ijms26146751. Int J Mol Sci. 2025. PMID: 40724999 Free PMC article. Review.
-
Gut microbiota derived metabolite trimethylamine N-oxide influences prostate cancer progression via the p38/HMOX1 pathway.Front Pharmacol. 2025 Jan 9;15:1526051. doi: 10.3389/fphar.2024.1526051. eCollection 2024. Front Pharmacol. 2025. PMID: 39850572 Free PMC article.
References
-
- Nørgaard M, Jensen AØ, Jacobsen JB, Cetin K, Fryzek JP, Sørensen HT. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007) J Urol. 2010;184:162–7. - PubMed
-
- Nuhn P, De Bono JS, Fizazi K, Freedland SJ, Grilli M, Kantoff PW. et al. Update on systemic prostate cancer therapies: management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur Urol. 2019;75:88–99. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

