Well-defined alginate oligosaccharides ameliorate joint pain and inflammation in a mouse model of gouty arthritis
- PMID: 38855180
- PMCID: PMC11155397
- DOI: 10.7150/thno.95611
Well-defined alginate oligosaccharides ameliorate joint pain and inflammation in a mouse model of gouty arthritis
Abstract
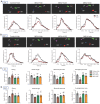
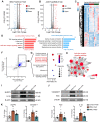
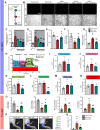
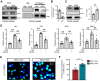
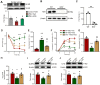
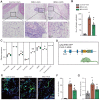
Background: Gouty arthritis causes severe pain and inflammation. Alginate oligosaccharides (AOSs) are natural products derived from alginate and have anti-inflammatory properties. We explored the potential effects of AOSs with different degrees of polymerization (Dp) on gouty arthritis and associated mechanisms. Methods: We established a mouse model of gouty arthritis by injecting monosodium urate (MSU) into ankle joint. Nocifensive behavior, gait and ankle swelling were used to study AOS's effects. Biochemical assays, in vivo imaging, live cell Ca2+ imaging, electrophysiology, RNA-sequencing, etc. were used for mechanism exploration. Results: AOS2 (Dp=2), AOS3 (Dp=3) and AOS4 (Dp=4) all inhibited ankle swelling, whereas AOS2&3 produced the most obvious analgesia on model mice. AOS3, which was picked for further evaluation, produced dose-dependent ameliorative effects on model mice. AOS3 reversed gait impairments but did not alter locomotor activity. AOS3 inhibited NLRP3 inflammasome activation and inflammatory cytokine up-regulation in ankle joint. AOS3 ameliorated MSU-induced oxidative stress and reactive oxygen species (ROS) production both in vivo and in vitro and reversed the impaired mitochondrial bioenergetics. AOS3 activated the Nrf2 pathway and promoted Nrf2 disassociation from Keap1-bound complex and Nrf2 nuclear translocation, thus facilitating antioxidant gene expression via Nrf2-dependent mechanism. Nrf2 gene deficiency abolished AOS3's ameliorative effects on pain, inflammation and oxidative stress in ankle joints of model mice. AOS3 reduced TRPV1 functional enhancement in DRG neurons and constrained neuroactive peptide release. Conclusions: AOS3 ameliorates gouty arthritis via activating Nrf2-dependent antioxidant signaling, resulting in suppression of ROS-mediated NLRP3 inflammasome activation and TRPV1 enhancement. AOS3 may be novel therapeutics for gouty arthritis.
Keywords: Inflammation; NLRP3; Neuropeptide; Oligosaccharides; ROS; TRPV1.
© The author(s).
Conflict of interest statement
Competing Interests: Dr. Zhende Liu is one of the inventors on patent applications related to this work filed by Haitang (Jiangsu) Biotechnology Co, Ltd (ZL 202110831844.0 and PCT/CN2021/107883). All other authors declare that they have no competing interests.
Figures



Similar articles
-
Phillyrin inhibits oxidative stress and neutrophil extracellular trap formation through the KEAP1/NRF2 pathway in gouty arthritis.Immunol Res. 2024 Dec;72(6):1489-1501. doi: 10.1007/s12026-024-09548-8. Epub 2024 Oct 22. Immunol Res. 2024. PMID: 39436625
-
Curcumin ameliorates monosodium urate-induced gouty arthritis through Nod-like receptor 3 inflammasome mediation via inhibiting nuclear factor-kappa B signaling.J Cell Biochem. 2019 Apr;120(4):6718-6728. doi: 10.1002/jcb.27969. Epub 2018 Dec 28. J Cell Biochem. 2019. PMID: 30592318
-
Gallic Acid Alleviates Gouty Arthritis by Inhibiting NLRP3 Inflammasome Activation and Pyroptosis Through Enhancing Nrf2 Signaling.Front Immunol. 2020 Dec 7;11:580593. doi: 10.3389/fimmu.2020.580593. eCollection 2020. Front Immunol. 2020. PMID: 33365024 Free PMC article.
-
Activation of Nrf2 antioxidant signaling alleviates gout arthritis pain and inflammation.Biomed Pharmacother. 2024 Jan;170:115957. doi: 10.1016/j.biopha.2023.115957. Epub 2023 Dec 1. Biomed Pharmacother. 2024. PMID: 38042115
-
Mechanism of Reactive Oxygen Species-Guided Immune Responses in Gouty Arthritis and Potential Therapeutic Targets.Biomolecules. 2024 Aug 9;14(8):978. doi: 10.3390/biom14080978. Biomolecules. 2024. PMID: 39199366 Free PMC article. Review.
Cited by
-
Neutrophil Extracellular Trap Formation Model Induced by Monosodium Urate and Phorbol Myristate Acetate: Involvement in MAPK Signaling Pathways.Int J Mol Sci. 2024 Dec 27;26(1):143. doi: 10.3390/ijms26010143. Int J Mol Sci. 2024. PMID: 39796001 Free PMC article.
-
Moderation of cross linkage of sodium alginate-polyethylene oxide films loaded with natamycin for treatment of Aspergillus fumigatus keratitis.J Mater Sci Mater Med. 2025 Jun 6;36(1):48. doi: 10.1007/s10856-025-06900-8. J Mater Sci Mater Med. 2025. PMID: 40478454 Free PMC article.
-
HNF-1β alleviates podocyte injury in lupus nephritis by maintaining endoplasmic reticulum homeostasis.Lupus Sci Med. 2024 Nov 27;11(2):e001349. doi: 10.1136/lupus-2024-001349. Lupus Sci Med. 2024. PMID: 39608815 Free PMC article.
-
Meridian-Sinew Release Therapy as an adjunctive treatment for gouty arthritis: a systematic review and meta-analysis.J Orthop Surg Res. 2025 Aug 18;20(1):769. doi: 10.1186/s13018-025-06123-1. J Orthop Surg Res. 2025. PMID: 40826358 Free PMC article.
-
Local oxygen concentration reversal from hyperoxia to hypoxia monitored by optical-resolution photoacoustic microscopy in inflammation-resolution process.Photoacoustics. 2025 May 17;44:100730. doi: 10.1016/j.pacs.2025.100730. eCollection 2025 Aug. Photoacoustics. 2025. PMID: 40510562 Free PMC article.
References
-
- Dalbeth N, Choi HK, Joosten LAB, Khanna PP, Matsuo H, Perez-Ruiz F. et al. Gout. Nat Rev Dis Primers. 2019;5:69. - PubMed
-
- Dalbeth N, Haskard DO. Mechanisms of inflammation in gout. Rheumatology (Oxford) 2005;44:1090–6. - PubMed
-
- Terkeltaub R. Update on gout: new therapeutic strategies and options. Nat Rev Rheumatol. 2010;6:30–8. - PubMed
-
- Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol. 2020;16:380–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

