Microfluidic technologies for advanced antimicrobial susceptibility testing
- PMID: 38855477
- PMCID: PMC11162290
- DOI: 10.1063/5.0190112
Microfluidic technologies for advanced antimicrobial susceptibility testing
Abstract
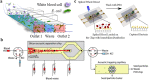
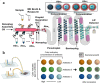
Antimicrobial resistance is getting serious and becoming a threat to public health worldwide. The improper and excessive use of antibiotics is responsible for this situation. The standard methods used in clinical laboratories, to diagnose bacterial infections, identify pathogens, and determine susceptibility profiles, are time-consuming and labor-intensive, leaving the empirical antimicrobial therapy as the only option for the first treatment. To prevent the situation from getting worse, evidence-based therapy should be given. The choosing of effective drugs requires powerful diagnostic tools to provide comprehensive information on infections. Recent progress in microfluidics is pushing infection diagnosis and antimicrobial susceptibility testing (AST) to be faster and easier. This review summarizes the recent development in microfluidic assays for rapid identification and AST in bacterial infections. Finally, we discuss the perspective of microfluidic-AST to develop the next-generation infection diagnosis technologies.
© 2024 Author(s).
Conflict of interest statement
The authors have no conflicts to disclose.
Figures




Similar articles
-
Combating Antimicrobial Resistance via Single-Cell Diagnostic Technologies Powered by Droplet Microfluidics.Acc Chem Res. 2022 Jan 18;55(2):123-133. doi: 10.1021/acs.accounts.1c00462. Epub 2021 Dec 13. Acc Chem Res. 2022. PMID: 34898173 Free PMC article.
-
Microfluidic Systems for Antimicrobial Susceptibility Testing.Adv Biochem Eng Biotechnol. 2022;179:291-309. doi: 10.1007/10_2021_164. Adv Biochem Eng Biotechnol. 2022. PMID: 33851232
-
Identification and Antimicrobial Susceptibility Testing of Campylobacter Using a Microfluidic Lab-on-a-Chip Device.Appl Environ Microbiol. 2020 Apr 17;86(9):e00096-20. doi: 10.1128/AEM.00096-20. Print 2020 Apr 17. Appl Environ Microbiol. 2020. PMID: 32111591 Free PMC article.
-
Rapid Phenotypic and Genotypic Antimicrobial Susceptibility Testing Approaches for Use in the Clinical Laboratory.Antibiotics (Basel). 2024 Aug 22;13(8):786. doi: 10.3390/antibiotics13080786. Antibiotics (Basel). 2024. PMID: 39200086 Free PMC article. Review.
-
Nucleic acid amplification-based microfluidic approaches for antimicrobial susceptibility testing.Analyst. 2021 May 17;146(10):3101-3113. doi: 10.1039/d1an00180a. Analyst. 2021. PMID: 33876805 Review.
Cited by
-
Nucleic acid amplification tests in digital microfluidics: the promise of next-generation point-of-care diagnostics.Microsyst Nanoeng. 2025 Aug 18;11(1):155. doi: 10.1038/s41378-025-00977-5. Microsyst Nanoeng. 2025. PMID: 40825819 Free PMC article. Review.
References
-
- Ventola C. L., Pharm. Ther. 40(4), 277–283 (2015); available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4378521.
-
- O’Neill J., Tackling Drug-Resistant Infections Globally: Final Report and Recommendations (HM Government, 2016); available at https://amr-review.org/sites/default/files/160525_Final%20paper_with%20c....
-
- Centers for Disease Control and Prevention, Antibiotic Resistance Threats in the United States (CDC, 2013), p.1–114; available at https://stacks.cdc.gov/view/cdc/20705/cdc_20705_DS1.pdf.
Publication types
LinkOut - more resources
Full Text Sources
