Analysis of Clinical Trials Using Anti-Tumor Traditional Chinese Medicine Monomers
- PMID: 38855536
- PMCID: PMC11162644
- DOI: 10.2147/DDDT.S454774
Analysis of Clinical Trials Using Anti-Tumor Traditional Chinese Medicine Monomers
Abstract
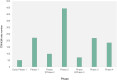
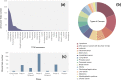
The potential anti-cancer effect of traditional Chinese medicine (TCM) monomers has been widely studied due to their advantages of well-defined structure, clear therapeutic effects, and easy quality control during the manufacturing process. However, clinical trial information on these monomers is scarce, resulting in a lack of knowledge regarding the research progress, efficacy, and adverse reactions at the clinical stage. Therefore, this study systematically reviewed the clinical trials on the anti-cancer effect of TCM monomers registered in the Clinicaltrials.gov website before 2023.4.30, paying special attention to the trials on tumors, aiming to explore the research results and development prospects in this field. A total of 1982 trials were started using 69 of the 131 TCM monomers. The number of clinical trials performed each year showed an overall upward trend. However, only 26 monomers entered into 519 interventional anti-tumor trials, with vinblastine (194, 37.38%) and camptothecin (146, 28.13%) being the most used. A total of 45 tumors were studied in these 519 trials, with lymphoma (112, 21.58%) being the most frequently studied. Clinical trials are also unevenly distributed across locations and sponsors/collaborators. The location and the sponsor/collaborator with the highest number of performed trials were the United States (651,32.85%) and NIH (77). Therefore, China and its institutions still have large room for progress in promoting TCM monomers in anti-tumor clinical trials. In the next step, priority should be given to the improvement of the research and development ability of domestic enterprises, universities and other institutions, using modern scientific and technological means to solve the problems of poor water solubility and strong toxic and side effects of monomers, so as to promote the clinical research of TCM monomers.
Keywords: adverse reactions; anti-cancer; interventional clinical trials; research progress; traditional Chinese medicine monomers.
© 2024 Lv et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures





Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Rabdosia rubescens (Hemsl.) H. Hara: A potent anti-tumor herbal remedy - Botany, phytochemistry, and clinical applications and insights.J Ethnopharmacol. 2025 Jan 31;340:119200. doi: 10.1016/j.jep.2024.119200. Epub 2024 Dec 3. J Ethnopharmacol. 2025. PMID: 39631716
-
Oral traditional Chinese medication for adhesive small bowel obstruction.Cochrane Database Syst Rev. 2012 May 16;2012(5):CD008836. doi: 10.1002/14651858.CD008836.pub2. Cochrane Database Syst Rev. 2012. PMID: 22592734 Free PMC article.
-
Chinese herbal medicine for oesophageal cancer.Cochrane Database Syst Rev. 2016 Jan 22;2016(1):CD004520. doi: 10.1002/14651858.CD004520.pub7. Cochrane Database Syst Rev. 2016. PMID: 26799001 Free PMC article.
Cited by
-
The tumor microenvironment in hepatocellular carcinoma: mechanistic insights and therapeutic potential of traditional Chinese medicine.Mol Cancer. 2025 Jun 10;24(1):173. doi: 10.1186/s12943-025-02378-8. Mol Cancer. 2025. PMID: 40495147 Free PMC article. Review.
-
Advancing cancer therapy: Nanomaterial-based encapsulation strategies for enhanced delivery and efficacy of curcumin.Mater Today Bio. 2025 Jun 9;33:101963. doi: 10.1016/j.mtbio.2025.101963. eCollection 2025 Aug. Mater Today Bio. 2025. PMID: 40575656 Free PMC article. Review.
-
Kang Ru enhances paclitaxel's efficacy against breast cancer progression.Am J Cancer Res. 2025 May 15;15(5):2180-2192. doi: 10.62347/XMGX2636. eCollection 2025. Am J Cancer Res. 2025. PMID: 40520866 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

