Neuregulin-1, a member of the epidermal growth factor family, mitigates STING-mediated pyroptosis and necroptosis in ischaemic flaps
- PMID: 38855574
- PMCID: PMC11162832
- DOI: 10.1093/burnst/tkae035
Neuregulin-1, a member of the epidermal growth factor family, mitigates STING-mediated pyroptosis and necroptosis in ischaemic flaps
Abstract
Background: Ensuring the survival of the distal end of a random flap during hypoperfusion (ischaemia) is difficult in clinical practice. Effective prevention of programmed cell death is a potential strategy for inhibiting ischaemic flap necrosis. The activation of stimulator of interferon genes (STING) pathway promotes inflammation and leads to cell death. The epidermal growth factor family member neuregulin-1 (NRG1) reduces cell death by activating the protein kinase B (AKT) signalling pathway. Moreover, AKT signalling negatively regulates STING activity. We aimed to verify the efficacy of NRG1 injection in protecting against flap necrosis. Additionally, we investigated whether NRG1 effectively enhances ischemic flap survival by inhibiting pyroptosis and necroptosis through STING suppression.
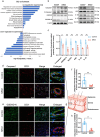
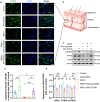
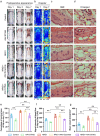
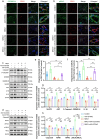
Methods: A random-pattern skin flap model was generated on the backs of C57BL/6 mice. The skin flap survival area was determined. The blood supply and vascular network of the flap was assessed by laser Doppler blood flow analysis. Cluster of differentiation 34 immunohistochemistry (IHC) and haematoxylin and eosin (H&E) staining of the flap sections revealed microvessels. Transcriptome sequencing analysis revealed the mechanism by which NRG1 promotes the survival of ischaemic flaps. The levels of angiogenesis, oxidative stress, necroptosis, pyroptosis and indicators associated with signalling pathways in flaps were examined by IHC, immunofluorescence and Western blotting. Packaging adeno-associated virus (AAV) was used to activate STING in flaps.
Results: NRG1 promoted the survival of ischaemic flaps. An increased subcutaneous vascular network and neovascularization were found in ischaemic flaps after the application of NRG1. Transcriptomic gene ontology enrichment analysis and protein level detection indicated that necroptosis, pyroptosis and STING activity were reduced in the NRG1 group. The phosphorylation of AKT and forkhead box O3a (FOXO3a) were increased after NRG1 treatment. The increased expression of STING in flaps induced by AAV reversed the therapeutic effect of NRG1. The ability of NRG1 to phosphorylate AKT-FOXO3a, inhibit STING and promote flap survival was abolished after the application of the AKT inhibitor MK2206.
Conclusions: NRG1 inhibits pyroptosis and necroptosis by activating the AKT-FOXO3a signalling pathway to suppress STING activation and promote ischaemic flap survival.
Keywords: Epidermal growth factor; Ischaemic flaps; Necroptosis; Neuregulin-1; Pyroptosis; STING.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
None declared.
Figures


Similar articles
-
Naringenin reduces oxidative stress and necroptosis, apoptosis, and pyroptosis in random-pattern skin flaps by enhancing autophagy.Eur J Pharmacol. 2024 May 5;970:176455. doi: 10.1016/j.ejphar.2024.176455. Epub 2024 Feb 27. Eur J Pharmacol. 2024. PMID: 38423240
-
Cyclic helix B peptide promotes random-pattern skin flap survival via TFE3-mediated enhancement of autophagy and reduction of ROS levels.Br J Pharmacol. 2022 Jan;179(2):301-321. doi: 10.1111/bph.15702. Epub 2021 Dec 14. Br J Pharmacol. 2022. PMID: 34622942
-
Inhibition of PLA2G4E/cPLA2 promotes survival of random skin flaps by alleviating Lysosomal membrane permeabilization-Induced necroptosis.Autophagy. 2022 Aug;18(8):1841-1863. doi: 10.1080/15548627.2021.2002109. Epub 2021 Dec 7. Autophagy. 2022. PMID: 34872436 Free PMC article.
-
Emerging role of STING signalling in CNS injury: inflammation, autophagy, necroptosis, ferroptosis and pyroptosis.J Neuroinflammation. 2022 Oct 4;19(1):242. doi: 10.1186/s12974-022-02602-y. J Neuroinflammation. 2022. PMID: 36195926 Free PMC article. Review.
-
Analysis on the pathogenesis and treatment progress of NRG1 fusion-positive non-small cell lung cancer.Front Oncol. 2024 Jun 18;14:1405380. doi: 10.3389/fonc.2024.1405380. eCollection 2024. Front Oncol. 2024. PMID: 38957319 Free PMC article. Review.
Cited by
-
The interplay of p16INK4a and non-coding RNAs: bridging cellular senescence, aging, and cancer.Biogerontology. 2025 Feb 5;26(2):50. doi: 10.1007/s10522-025-10194-2. Biogerontology. 2025. PMID: 39907830 Review.
-
Microglial Modulation in Alzheimer's Disease: Central Players in Neuroinflammation and Pathogenesis.Curr Alzheimer Res. 2025;22(1):56-82. doi: 10.2174/0115672050364292250113063513. Curr Alzheimer Res. 2025. PMID: 39976100 Review.
-
Cytokines in age-related eye diseases: pathogenesis and potential targets for innovative therapies.Naunyn Schmiedebergs Arch Pharmacol. 2025 Aug;398(8):9371-9386. doi: 10.1007/s00210-025-03926-1. Epub 2025 Mar 1. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40021512 Review.
-
Cu-DHM nanozymes treat flap ischemia-reperfusion injury by amplifying immune modulation in a cascade manner and inhibiting cell apoptosis.Bioact Mater. 2025 Jun 24;51:720-739. doi: 10.1016/j.bioactmat.2025.06.036. eCollection 2025 Sep. Bioact Mater. 2025. PMID: 40641838 Free PMC article.
-
Novel cardiac biomarkers and multiple-marker approach in the early detection, prognosis, and risk stratification of cardiac diseases.World J Cardiol. 2025 Jul 26;17(7):106561. doi: 10.4330/wjc.v17.i7.106561. World J Cardiol. 2025. PMID: 40741019 Free PMC article. Review.
References
-
- Charafeddine AH, Drake R, McBride J. et al. Facelift: History and Anatomy. Clin Plast Surg 2019;46:505–13. - PubMed
-
- Summers BK, Siegle RJ. Facial cutaneous reconstructive surgery: general aesthetic principles. J Am Acad Dermatol 1993;29:669–81. - PubMed
-
- Chung S, Hazen A, Levine JP. et al. Vascularized acellular dermal matrix island flaps for the repair of abdominal muscle defects. Plast Reconstr Surg 2003;111:225–32. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

