Two-photon autofluorescence lifetime assay of rabbit photoreceptors and retinal pigment epithelium during light-dark visual cycles in rabbit retina
- PMID: 38855698
- PMCID: PMC11161359
- DOI: 10.1364/BOE.511806
Two-photon autofluorescence lifetime assay of rabbit photoreceptors and retinal pigment epithelium during light-dark visual cycles in rabbit retina
Abstract
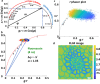
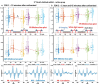
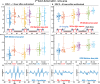
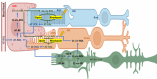
Two-photon excited fluorescence (TPEF) is a powerful technique that enables the examination of intrinsic retinal fluorophores involved in cellular metabolism and the visual cycle. Although previous intensity-based TPEF studies in non-human primates have successfully imaged several classes of retinal cells and elucidated aspects of both rod and cone photoreceptor function, fluorescence lifetime imaging (FLIM) of the retinal cells under light-dark visual cycle has yet to be fully exploited. Here we demonstrate a FLIM assay of photoreceptors and retinal pigment epithelium (RPE) that reveals key insights into retinal physiology and adaptation. We found that photoreceptor fluorescence lifetimes increase and decrease in sync with light and dark exposure, respectively. This is likely due to changes in all-trans-retinol and all-trans-retinal levels in the outer segments, mediated by phototransduction and visual cycle activity. During light exposure, RPE fluorescence lifetime was observed to increase steadily over time, as a result of all-trans-retinol accumulation during the visual cycle and decreasing metabolism caused by the lack of normal perfusion of the sample. Our system can measure the fluorescence lifetime of intrinsic retinal fluorophores on a cellular scale, revealing differences in lifetime between retinal cell classes under different conditions of light and dark exposure.
© 2024 Optica Publishing Group.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Rattner A., Smallwood P. M., Nathans J., et al. , “Identification and Characterization of All-trans-retinol Dehydrogenase from Photoreceptor Outer Segments, the Visual Cycle Enzyme That Reduces All-trans-retinal to All-trans-retinol,” J. Biol. Chem. 275(15), 11034–11043 (2000). 10.1074/jbc.275.15.11034 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
