Optimizing the Biocompatibility of PLLA Stent Materials: Strategy with Biomimetic Coating
- PMID: 38855731
- PMCID: PMC11162223
- DOI: 10.2147/IJN.S462691
Optimizing the Biocompatibility of PLLA Stent Materials: Strategy with Biomimetic Coating
Abstract
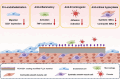
Background: Poly-L-lactic acid (PLLA) stents have broad application prospects in the treatment of cardiovascular diseases due to their excellent mechanical properties and biodegradability. However, foreign body reactions caused by stent implantation remain a bottleneck that limits the clinical application of PLLA stents. To solve this problem, the biocompatibility of PLLA stents must be urgently improved. Albumin, the most abundant inert protein in the blood, possesses the ability to modify the surface of biomaterials, mitigating foreign body reactions-a phenomenon described as the "stealth effect". In recent years, a strategy based on albumin camouflage has become a focal point in nanomedicine delivery and tissue engineering research. Therefore, albumin surface modification is anticipated to enhance the surface biological characteristics required for vascular stents. However, the therapeutic applicability of this modification has not been fully explored.
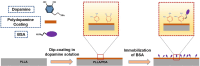
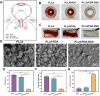
Methods: Herein, a bionic albumin (PDA-BSA) coating was constructed on the surface of PLLA by a mussel-inspired surface modification technique using polydopamine (PDA) to enhance the immobilization of bovine serum albumin (BSA).
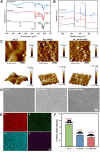
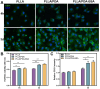
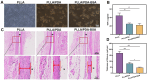
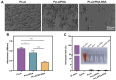
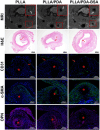
Results: Surface characterization revealed that the PDA-BSA coating was successfully constructed on the surface of PLLA materials, significantly improving their hydrophilicity. Furthermore, in vivo and in vitro studies demonstrated that this PDA-BSA coating enhanced the anticoagulant properties and pro-endothelialization effects of the PLLA material surface while inhibiting the inflammatory response and neointimal hyperplasia at the implantation site.
Conclusion: These findings suggest that the PDA-BSA coating provides a multifunctional biointerface for PLLA stent materials, markedly improving their biocompatibility. Further research into the diverse applications of this coating in vascular implants is warranted.
Keywords: Poly-L-lactic acid; albumin; biomimetic coating; stealth effect; vascular stent.
© 2024 Du et al.
Conflict of interest statement
The authors reports no conflicts of interest in this work.
Figures
Similar articles
-
Hemocompatibility and selective cell fate of polydopamine-assisted heparinized PEO/PLLA composite coating on biodegradable AZ31 alloy.Colloids Surf B Biointerfaces. 2014 Sep 1;121:451-60. doi: 10.1016/j.colsurfb.2014.06.036. Epub 2014 Jun 21. Colloids Surf B Biointerfaces. 2014. PMID: 25009102
-
New stent surface materials: the impact of polymer-dependent interactions of human endothelial cells, smooth muscle cells, and platelets.Acta Biomater. 2014 Feb;10(2):688-700. doi: 10.1016/j.actbio.2013.10.015. Epub 2013 Oct 19. Acta Biomater. 2014. PMID: 24148751
-
High-Efficient Vacuum Ultraviolet-Ozone Assist-Deposited Polydopamine for Poly(lactic-co-glycolic acid)-Coated Pure Zn toward Biodegradable Cardiovascular Stent Applications.ACS Appl Mater Interfaces. 2022 Jan 19;14(2):3536-3550. doi: 10.1021/acsami.1c21567. Epub 2021 Dec 23. ACS Appl Mater Interfaces. 2022. PMID: 34941257
-
Structure, properties and applications of mussel-inspired polydopamine.J Biomed Nanotechnol. 2014 Oct;10(10):3063-84. doi: 10.1166/jbn.2014.1888. J Biomed Nanotechnol. 2014. PMID: 25992429 Review.
-
Current Advances in the Utilization of Polydopamine Nanostructures in Biomedical Therapy.Biotechnol J. 2019 Dec;14(12):e1900080. doi: 10.1002/biot.201900080. Epub 2019 Aug 8. Biotechnol J. 2019. PMID: 31293058 Review.
Cited by
-
An injectable multifunctional nanocomposite hydrogel promotes vascularized bone regeneration by regulating macrophages.J Nanobiotechnology. 2025 Apr 7;23(1):283. doi: 10.1186/s12951-025-03358-2. J Nanobiotechnology. 2025. PMID: 40197239 Free PMC article.
-
Dose-dependent enhancement of in vitro osteogenic activity on strontium-decorated polyetheretherketone.Sci Rep. 2025 Jan 24;15(1):3063. doi: 10.1038/s41598-025-86561-3. Sci Rep. 2025. PMID: 39856116 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

