Glycolytic lactate in diabetic kidney disease
- PMID: 38855868
- PMCID: PMC11382878
- DOI: 10.1172/jci.insight.168825
Glycolytic lactate in diabetic kidney disease
Abstract
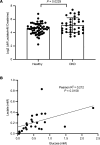
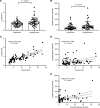
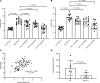
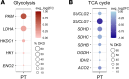
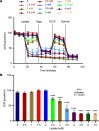
Lactate elevation is a well-characterized biomarker of mitochondrial dysfunction, but its role in diabetic kidney disease (DKD) is not well defined. Urine lactate was measured in patients with type 2 diabetes (T2D) in 3 cohorts (HUNT3, SMART2D, CRIC). Urine and plasma lactate were measured during euglycemic and hyperglycemic clamps in participants with type 1 diabetes (T1D). Patients in the HUNT3 cohort with DKD had elevated urine lactate levels compared with age- and sex-matched controls. In patients in the SMART2D and CRIC cohorts, the third tertile of urine lactate/creatinine was associated with more rapid estimated glomerular filtration rate decline, relative to first tertile. Patients with T1D demonstrated a strong association between glucose and lactate in both plasma and urine. Glucose-stimulated lactate likely derives in part from proximal tubular cells, since lactate production was attenuated with sodium-glucose cotransporter-2 (SGLT2) inhibition in kidney sections and in SGLT2-deficient mice. Several glycolytic genes were elevated in human diabetic proximal tubules. Lactate levels above 2.5 mM potently inhibited mitochondrial oxidative phosphorylation in human proximal tubule (HK2) cells. We conclude that increased lactate production under diabetic conditions can contribute to mitochondrial dysfunction and become a feed-forward component to DKD pathogenesis.
Keywords: Chronic kidney disease; Diabetes; Mitochondria; Nephrology.
Figures

References
-
- Sharma K, Ziyadeh FN. Biochemical events and cytokine interactions linking glucose metabolism to the development of diabetic nephropathy. Semin Nephrol. 1997;17(2):80–92. - PubMed
-
- Greene D, et al. Role of sorbitol accumulation and myo-inositol depletion in paranodal swelling of large myelinated nerve fibers in the insulin-deficient spontaneously diabetic bio-breeding rat. Reversal by insulin replacement, an aldose reductase inhibitor, and myo-inositol. J Clin Invest. 1987;79(5):1479–1485. doi: 10.1172/JCI112977. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- UH3 DK114907/DK/NIDDK NIH HHS/United States
- U54 DK137307/DK/NIDDK NIH HHS/United States
- UH3 DK114923/DK/NIDDK NIH HHS/United States
- UH3 DK114908/DK/NIDDK NIH HHS/United States
- U2C DK114886/DK/NIDDK NIH HHS/United States
- UH3 DK114915/DK/NIDDK NIH HHS/United States
- UH3 DK114920/DK/NIDDK NIH HHS/United States
- R01 DK110541/DK/NIDDK NIH HHS/United States
- UH3 DK114861/DK/NIDDK NIH HHS/United States
- R01 DK112042/DK/NIDDK NIH HHS/United States
- I01 BX001340/BX/BLRD VA/United States
- UH3 DK114866/DK/NIDDK NIH HHS/United States
- R01 HL142814/HL/NHLBI NIH HHS/United States
- DP3 DK094352/DK/NIDDK NIH HHS/United States
- RF1 AG061296/AG/NIA NIH HHS/United States
- U01 DK114920/DK/NIDDK NIH HHS/United States
- U24 DK060990/DK/NIDDK NIH HHS/United States
- R01 DK106102/DK/NIDDK NIH HHS/United States
- UH3 DK114926/DK/NIDDK NIH HHS/United States
- UH3 DK114937/DK/NIDDK NIH HHS/United States
- P30 DK079337/DK/NIDDK NIH HHS/United States
- UH3 DK114870/DK/NIDDK NIH HHS/United States
- R43 DK130732/DK/NIDDK NIH HHS/United States
- UH3 DK114933/DK/NIDDK NIH HHS/United States

