SFTPB in serum extracellular vesicles as a biomarker of progressive pulmonary fibrosis
- PMID: 38855869
- PMCID: PMC11382876
- DOI: 10.1172/jci.insight.177937
SFTPB in serum extracellular vesicles as a biomarker of progressive pulmonary fibrosis
Abstract
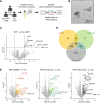
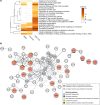
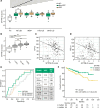
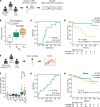
Progressive pulmonary fibrosis (PPF), defined as the worsening of various interstitial lung diseases (ILDs), currently lacks useful biomarkers. To identify novel biomarkers for early detection of patients at risk of PPF, we performed a proteomic analysis of serum extracellular vesicles (EVs). Notably, the identified candidate biomarkers were enriched for lung-derived proteins participating in fibrosis-related pathways. Among them, pulmonary surfactant-associated protein B (SFTPB) in serum EVs could predict ILD progression better than the known biomarkers, serum KL-6 and SP-D, and it was identified as an independent prognostic factor from ILD-gender-age-physiology index. Subsequently, the utility of SFTPB for predicting ILD progression was evaluated further in 2 cohorts using serum EVs and serum, respectively, suggesting that SFTPB in serum EVs but not in serum was helpful. Among SFTPB forms, pro-SFTPB levels were increased in both serum EVs and lungs of patients with PPF compared with those of the control. Consistently, in a mouse model, the levels of pro-SFTPB, primarily originating from alveolar epithelial type 2 cells, were increased similarly in serum EVs and lungs, reflecting pro-fibrotic changes in the lungs, as supported by single-cell RNA sequencing. SFTPB, especially its pro-form, in serum EVs could serve as a biomarker for predicting ILD progression.
Keywords: Fibrosis; Proteomics; Pulmonary surfactants; Pulmonology.
Figures

References
-
- Travis WD, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

