Cytomegalovirus infection lengthens the cell cycle of granule cell precursors during postnatal cerebellar development
- PMID: 38855871
- PMCID: PMC11382886
- DOI: 10.1172/jci.insight.175525
Cytomegalovirus infection lengthens the cell cycle of granule cell precursors during postnatal cerebellar development
Abstract
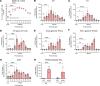
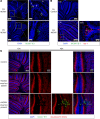
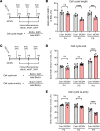
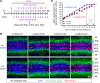
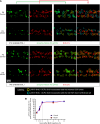
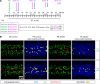
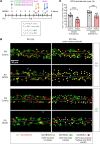
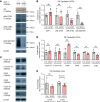
Human cytomegalovirus (HCMV) infection in infants infected in utero can lead to a variety of neurodevelopmental disorders. However, mechanisms underlying altered neurodevelopment in infected infants remain poorly understood. We have previously described a murine model of congenital HCMV infection in which murine CMV (MCMV) spreads hematogenously and establishes a focal infection in all regions of the brain of newborn mice, including the cerebellum. Infection resulted in disruption of cerebellar cortical development characterized by reduced cerebellar size and foliation. This disruption was associated with altered cell cycle progression of the granule cell precursors (GCPs), which are the progenitors that give rise to granule cells (GCs), the most abundant neurons in the cerebellum. In the current study, we have demonstrated that MCMV infection leads to prolonged GCP cell cycle, premature exit from the cell cycle, and reduced numbers of GCs resulting in cerebellar hypoplasia. Treatment with TNF-α neutralizing antibody partially normalized the cell cycle alterations of GCPs and altered cerebellar morphogenesis induced by MCMV infection. Collectively, our results argue that virus-induced inflammation altered the cell cycle of GCPs resulting in a reduced numbers of GCs and cerebellar cortical hypoplasia, thus providing a potential mechanism for altered neurodevelopment in fetuses infected with HCMV.
Keywords: Inflammation; Neurological disorders; Neuroscience.
Conflict of interest statement
Figures