Electrifying Hydroformylation Catalysts Exposes Voltage-Driven C-C Bond Formation
- PMID: 38856020
- PMCID: PMC11191585
- DOI: 10.1021/jacs.4c02992
Electrifying Hydroformylation Catalysts Exposes Voltage-Driven C-C Bond Formation
Abstract
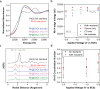
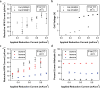
Electrochemical reactions can access a significant range of driving forces under operationally mild conditions and are thus envisioned to play a key role in decarbonizing chemical manufacturing. However, many reactions with well-established thermochemical precedents remain difficult to achieve electrochemically. For example, hydroformylation (thermo-HFN) is an industrially important reaction that couples olefins and carbon monoxide (CO) to make aldehydes. However, the electrochemical analogue of hydroformylation (electro-HFN), which uses protons and electrons instead of hydrogen gas, represents a complex C-C bond-forming reaction that is difficult to achieve at heterogeneous electrocatalysts. In this work, we import Rh-based thermo-HFN catalysts onto electrode surfaces to unlock electro-HFN reactivity. At mild conditions of room temperature and 5 bar CO, we achieve Faradaic efficiencies of up to 15% and turnover frequencies of up to 0.7 h-1. This electro-HFN rate is an order of magnitude greater than the corresponding thermo-HFN rate at the same catalyst, temperature, and pressure. Reaction kinetics and operando X-ray absorption spectroscopy provide evidence for an electro-HFN mechanism that involves distinct elementary steps relative to thermo-HFN. This work demonstrates a step-by-step experimental strategy for electrifying a well-studied thermochemical reaction to unveil a new electrocatalyst for a complex and underexplored electrochemical reaction.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Schiffer Z. J.; Manthiram K. Electrification and Decarbonization of the Chemical Industry. Joule 2017, 1 (1), 10–14. 10.1016/j.joule.2017.07.008. - DOI
-
- Mallapragada D. S.; Dvorkin Y.; Modestino M. A.; Esposito D. V.; Smith W. A.; Hodge B. M.; Harold M. P.; Donnelly V. M.; Nuz A.; Bloomquist C.; Baker K.; Grabow L. C.; Yan Y.; Rajput N. N.; Hartman R. L.; Biddinger E. J.; Aydil E. S.; Taylor A. D. Decarbonization of the Chemical Industry through Electrification: Barriers and Opportunities. Joule 2023, 7 (1), 23–41. 10.1016/j.joule.2022.12.008. - DOI
-
- Weissermel K.; Arpe H.-J.. Industrial Organic Chemistry, 4th ed.; WILEY-VCH Verlag GmbH & Co. KGaA, 2003.
LinkOut - more resources
Full Text Sources

