Bacterial cell volume regulation and the importance of cyclic di-AMP
- PMID: 38856222
- PMCID: PMC11332354
- DOI: 10.1128/mmbr.00181-23
Bacterial cell volume regulation and the importance of cyclic di-AMP
Abstract
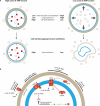
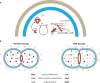
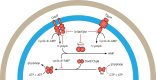
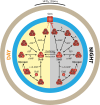
SUMMARYNucleotide-derived second messengers are present in all domains of life. In prokaryotes, most of their functionality is associated with general lifestyle and metabolic adaptations, often in response to environmental fluctuations of physical parameters. In the last two decades, cyclic di-AMP has emerged as an important signaling nucleotide in many prokaryotic lineages, including Firmicutes, Actinobacteria, and Cyanobacteria. Its importance is highlighted by the fact that both the lack and overproduction of cyclic di-AMP affect viability of prokaryotes that utilize cyclic di-AMP, and that it generates a strong innate immune response in eukaryotes. In bacteria that produce the second messenger, most molecular targets of cyclic di-AMP are associated with cell volume control. Besides, other evidence links the second messenger to cell wall remodeling, DNA damage repair, sporulation, central metabolism, and the regulation of glycogen turnover. In this review, we take a biochemical, quantitative approach to address the main cellular processes that are directly regulated by cyclic di-AMP and show that these processes are very connected and require regulation of a similar set of proteins to which cyclic di-AMP binds. Altogether, we argue that cyclic di-AMP is a master regulator of cell volume and that other cellular processes can be connected with cyclic di-AMP through this core function. We further highlight important directions in which the cyclic di-AMP field has to develop to gain a full understanding of the cyclic di-AMP signaling network and why some processes are regulated, while others are not.
Keywords: cell volume regulation; cell wall metabolism; cyclic di-AMP; osmoregulation; potassium and compatible solute transport; second messenger; signaling nucleotide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The second messenger c-di-AMP mediates bacterial exopolysaccharide biosynthesis: a review.Mol Biol Rep. 2020 Nov;47(11):9149-9157. doi: 10.1007/s11033-020-05930-5. Epub 2020 Oct 30. Mol Biol Rep. 2020. PMID: 33128205 Review.
-
A jack of all trades: the multiple roles of the unique essential second messenger cyclic di-AMP.Mol Microbiol. 2015 Jul;97(2):189-204. doi: 10.1111/mmi.13026. Epub 2015 May 9. Mol Microbiol. 2015. PMID: 25869574 Review.
-
Replenishing the cyclic-di-AMP pool: regulation of diadenylate cyclase activity in bacteria.Curr Genet. 2016 Nov;62(4):731-738. doi: 10.1007/s00294-016-0600-8. Epub 2016 Apr 13. Curr Genet. 2016. PMID: 27074767 Review.
-
Sustained Control of Pyruvate Carboxylase by the Essential Second Messenger Cyclic di-AMP in Bacillus subtilis.mBio. 2021 Feb 22;13(1):e0360221. doi: 10.1128/mbio.03602-21. Epub 2022 Feb 8. mBio. 2021. PMID: 35130724 Free PMC article.
-
An Essential Poison: Synthesis and Degradation of Cyclic Di-AMP in Bacillus subtilis.J Bacteriol. 2015 Oct;197(20):3265-74. doi: 10.1128/JB.00564-15. Epub 2015 Aug 3. J Bacteriol. 2015. PMID: 26240071 Free PMC article.
Cited by
-
Cell wall stress in bacteria increases a nucleotide second messenger to reduce turgor.Nat Microbiol. 2025 Jul;10(7):1562-1563. doi: 10.1038/s41564-025-02029-0. Nat Microbiol. 2025. PMID: 40588590 No abstract available.
-
Cyclic-di-AMP modulates cellular turgor in response to defects in bacterial cell wall synthesis.Nat Microbiol. 2025 Jul;10(7):1698-1710. doi: 10.1038/s41564-025-02027-2. Epub 2025 Jun 17. Nat Microbiol. 2025. PMID: 40528006 Free PMC article.
-
c-di-AMP is an envoy of inflammation.Nat Chem Biol. 2025 Aug;21(8):1132-1133. doi: 10.1038/s41589-025-01931-2. Nat Chem Biol. 2025. PMID: 40419772 No abstract available.
-
Transformative strategies for saline soil restoration: Harnessing halotolerant microorganisms and advanced technologies.World J Microbiol Biotechnol. 2025 Apr 28;41(5):140. doi: 10.1007/s11274-025-04342-6. World J Microbiol Biotechnol. 2025. PMID: 40289223 Review.
-
Metals in Motion: Understanding Labile Metal Pools in Bacteria.Biochemistry. 2025 Jan 21;64(2):329-345. doi: 10.1021/acs.biochem.4c00726. Epub 2025 Jan 5. Biochemistry. 2025. PMID: 39755956 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

