Evinacumab in homozygous familial hypercholesterolaemia: long-term safety and efficacy
- PMID: 38856678
- PMCID: PMC11242450
- DOI: 10.1093/eurheartj/ehae325
Evinacumab in homozygous familial hypercholesterolaemia: long-term safety and efficacy
Erratum in
-
Correction to: Evinacumab in homozygous familial hypercholesterolaemia: long-term safety and efficacy.Eur Heart J. 2024 Oct 21;45(40):4314. doi: 10.1093/eurheartj/ehae594. Eur Heart J. 2024. PMID: 39302808 Free PMC article. No abstract available.
Abstract
Background and aims: Homozygous familial hypercholesterolaemia (HoFH) is a rare genetic disorder characterized by severely elevated LDL cholesterol (LDL-C) and premature atherosclerotic cardiovascular disease. In the pivotal Phase 3 HoFH trial (NCT03399786), evinacumab significantly decreased LDL-C in patients with HoFH. This study assesses the long-term safety and efficacy of evinacumab in adult and adolescent patients with HoFH.
Methods: In this open-label, single-arm, Phase 3 trial (NCT03409744), patients aged ≥12 years with HoFH who were evinacumab-naïve or had previously received evinacumab in other trials (evinacumab-continue) received intravenous evinacumab 15 mg/kg every 4 weeks with stable lipid-lowering therapy.
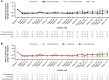
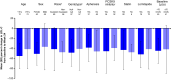
Results: A total of 116 patients (adults: n = 102; adolescents: n = 14) were enrolled, of whom 57 (49.1%) were female. Patients were treated for a median (range) duration of 104.3 (28.3-196.3) weeks. Overall, treatment-emergent adverse events (TEAEs) and serious TEAEs were reported in 93 (80.2%) and 27 (23.3%) patients, respectively. Two (1.7%) deaths were reported (neither was considered related to evinacumab). Three (2.6%) patients discontinued due to TEAEs (none were considered related to evinacumab). From baseline to Week 24, evinacumab decreased mean LDL-C by 43.6% [mean (standard deviation, SD), 3.4 (3.2) mmol/L] in the overall population; mean LDL-C reduction in adults and adolescents was 41.7% [mean (SD), 3.2 (3.3) mmol/L] and 55.4% [mean (SD), 4.7 (2.5) mmol/L], respectively.
Conclusions: In this large cohort of patients with HoFH, evinacumab was generally well tolerated and markedly decreased LDL-C irrespective of age and sex. Moreover, the efficacy and safety of evinacumab was sustained over the long term.
Keywords: Atherosclerosis; Cholesterol; Homozygous familial hypercholesterolaemia.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures


References
-
- Moorjani S, Roy M, Torres A, Betard C, Gagne C, Lambert M, et al. Mutations of low-density-lipoprotein-receptor gene, variation in plasma cholesterol, and expression of coronary heart disease in homozygous familial hypercholesterolaemia. Lancet 1993;341:1303–6. 10.1016/0140-6736(93)90815-X - DOI - PubMed
-
- Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J 2013;34:3478–90. 10.1093/eurheartj/eht273 - DOI - PMC - PubMed
-
- Cuchel M, Bruckert E, Ginsberg HN, Raal FJ, Santos RD, Hegele RA, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J 2014;35:2146–57. 10.1093/eurheartj/ehu274 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

